The comparison of outcomes from tyrosine kinase inhibitor monotherapy in second- or third-line for advanced non-small-cell lung cancer patients with wild-type or unknown EGFR status
- PMID: 26993607
- PMCID: PMC5094963
- DOI: 10.18632/oncotarget.8130
The comparison of outcomes from tyrosine kinase inhibitor monotherapy in second- or third-line for advanced non-small-cell lung cancer patients with wild-type or unknown EGFR status
Abstract
Background: Second-line treatment for advanced non-small-cell lung cancer (NSCLC) patients includes monotherapy with a third-generation cytotoxic drug (CT) or a tyrosine kinase inhibitor (TKI). These options are the actual standard for EGFR wild-type (WT) status, as patients with EGFR mutations achieve greater benefit by the use of TKI in first-line treatment. Some clinical trials and meta-analyses investigated the comparison between CT and TKI in second-line, but data are conflicting.
Methods: We designed a retrospective trial to gather information about TKI sensitivity in comparison with CT. We selected from clinical records patients treated with at least 1 line of CT and at least 1 line of TKI. We collected data about age, sex, performance status, comorbidity, smoking status, histotype, metastatic sites, EGFR status, treatment schedule, better response and time-to-progression (TTP) for each line of treatment and overall survival (OS).
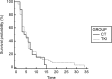
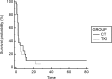
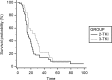
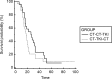
Results: 93 patients met selection criteria. Mean age 66,7 (range: 46-84). M/F ratio is 3:1. 39 EGFR-WT and 54 EGFR-UK. All patients received erlotinib or gefitinib as second-line treatment or erlotinib as third-line treatment. No TTP differences were observed for both second-line (HR:0,91; p = 0,6333) and third-line (HR:1.1; p = 0,6951) treatment (TKI vs CT). A trend of a benefit in OS in favor of 3rd-line TKI (HR:0,68; p = 0,11).
Conclusions: This study explores the role of TKIs in EGFR non-mutated NSCLC patients. OS analysis highlights a trend to a benefit in patients who received TKI in third-line, even if this result is statistically non-significant. Further analysis are needed to find an explanation for this observation.
Keywords: EGFR; chemotherapy; non-small-cell lung cancer; tyrosine kinase inhibitor.
Conflict of interest statement
The authors declare no potential conflicts of interests.
Figures
References
-
- Passiglia F, Bronte G, Castiglia M, Listì A, Calò V, Toia F, Cicero G, Fanale D, Rizzo S, Bazan V, Russo A. Prognostic and predictive biomarkers for targeted therapy in NSCLC: for whom the bell tolls? Expert Opin Biol Ther. 2015;15:1553–1566. - PubMed
-
- Bronte G, Rolfo C, Giovannetti E, Cicero G, Pauwels P, Passiglia F, Castiglia M, Rizzo S, Vullo FL, Fiorentino E, Van Meerbeeck J, Russo A. Are erlotinib and gefitinib interchangeable, opposite or complementary for non-small cell lung cancer treatment? Biological, pharmacological and clinical aspects. Crit Rev Oncol Hematol. 2014;89:300–313. - PubMed
-
- Rolfo C, Passiglia F, Castiglia M, Raez LE, Germonpre P, Gil-Bazo I, Zwaenepoel K, De Wilde A, Bronte G, Russo A, Van Meerbeeck JP, Van Schil P, Pauwels P. ALK and crizotinib: after the honeymoon…what else? Resistance mechanisms and new therapies to overcome it. Transl Lung Cancer Res. 2014;3:250–261. - PMC - PubMed
-
- Bronte G, Rizzo S, La Paglia L, Adamo V, Siragusa S, Ficorella C, Santini D, Bazan V, Colucci G, Gebbia N, Russo A. Driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma. Cancer Treat Rev. 2010;36:S21–29. - PubMed
-
- Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous