Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury
- PMID: 26993800
- PMCID: PMC5477508
- DOI: 10.1093/brain/aww039
Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury
Abstract
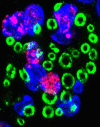
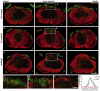
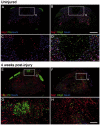
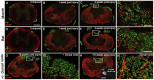
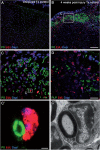
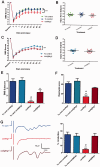
Following traumatic spinal cord injury, acute demyelination of spinal axons is followed by a period of spontaneous remyelination. However, this endogenous repair response is suboptimal and may account for the persistently compromised function of surviving axons. Spontaneous remyelination is largely mediated by Schwann cells, where demyelinated central axons, particularly in the dorsal columns, become associated with peripheral myelin. The molecular control, functional role and origin of these central remyelinating Schwann cells is currently unknown. The growth factor neuregulin-1 (Nrg1, encoded by NRG1) is a key signalling factor controlling myelination in the peripheral nervous system, via signalling through ErbB tyrosine kinase receptors. Here we examined whether Nrg1 is required for Schwann cell-mediated remyelination of central dorsal column axons and whether Nrg1 ablation influences the degree of spontaneous remyelination and functional recovery following spinal cord injury. In contused adult mice with conditional ablation of Nrg1, we found an absence of Schwann cells within the spinal cord and profound demyelination of dorsal column axons. There was no compensatory increase in oligodendrocyte remyelination. Removal of peripheral input to the spinal cord and proliferation studies demonstrated that the majority of remyelinating Schwann cells originated within the injured spinal cord. We also examined the role of specific Nrg1 isoforms, using mutant mice in which only the immunoglobulin-containing isoforms of Nrg1 (types I and II) were conditionally ablated, leaving the type III Nrg1 intact. We found that the immunoglobulin Nrg1 isoforms were dispensable for Schwann cell-mediated remyelination of central axons after spinal cord injury. When functional effects were examined, both global Nrg1 and immunoglobulin-specific Nrg1 mutants demonstrated reduced spontaneous locomotor recovery compared to injured controls, although global Nrg1 mutants were more impaired in tests requiring co-ordination, balance and proprioception. Furthermore, electrophysiological assessments revealed severely impaired axonal conduction in the dorsal columns of global Nrg1 mutants (where Schwann cell-mediated remyelination is prevented), but not immunoglobulin-specific mutants (where Schwann cell-mediated remyelination remains intact), providing robust evidence that the profound demyelinating phenotype observed in the dorsal columns of Nrg1 mutant mice is related to conduction failure. Our data provide novel mechanistic insight into endogenous regenerative processes after spinal cord injury, demonstrating that Nrg1 signalling regulates central axon remyelination and functional repair and drives the trans-differentiation of central precursor cells into peripheral nervous system-like Schwann cells that remyelinate spinal axons after injury. Manipulation of the Nrg1 system could therefore be exploited to enhance spontaneous repair after spinal cord injury and other central nervous system disorders with a demyelinating pathology.media-1vid110.1093/brain/aww039_video_abstractaww039_video_abstract.
Keywords: axon degeneration; demyelination; remyelination; spinal cord injury; transgenic model.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Similar articles
-
ErbB receptor signaling directly controls oligodendrocyte progenitor cell transformation and spontaneous remyelination after spinal cord injury.Glia. 2019 Jun;67(6):1036-1046. doi: 10.1002/glia.23586. Epub 2019 Jan 13. Glia. 2019. PMID: 30637799 Free PMC article.
-
Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination.Brain. 2013 Jul;136(Pt 7):2279-97. doi: 10.1093/brain/awt148. Brain. 2013. PMID: 23801741 Free PMC article.
-
Axonal neuregulin-1 regulates myelin sheath thickness.Science. 2004 Apr 30;304(5671):700-3. doi: 10.1126/science.1095862. Epub 2004 Mar 25. Science. 2004. PMID: 15044753
-
Demyelination in spinal cord injury and multiple sclerosis: what can we do to enhance functional recovery?J Neurotrauma. 1992 Mar;9 Suppl 1:S105-17. J Neurotrauma. 1992. PMID: 1588601 Review.
-
Neuregulin/ErbB Signaling in Developmental Myelin Formation and Nerve Repair.Curr Top Dev Biol. 2016;116:45-64. doi: 10.1016/bs.ctdb.2015.11.009. Epub 2016 Feb 1. Curr Top Dev Biol. 2016. PMID: 26970613 Review.
Cited by
-
Factors related to improved American Spinal Injury Association grade of acute traumatic spinal cord injury.World J Clin Cases. 2020 Oct 26;8(20):4807-4815. doi: 10.12998/wjcc.v8.i20.4807. World J Clin Cases. 2020. PMID: 33195648 Free PMC article.
-
ErbB receptor signaling directly controls oligodendrocyte progenitor cell transformation and spontaneous remyelination after spinal cord injury.Glia. 2019 Jun;67(6):1036-1046. doi: 10.1002/glia.23586. Epub 2019 Jan 13. Glia. 2019. PMID: 30637799 Free PMC article.
-
Secretion of a mammalian chondroitinase ABC aids glial integration at PNS/CNS boundaries.Sci Rep. 2020 Jul 9;10(1):11262. doi: 10.1038/s41598-020-67526-0. Sci Rep. 2020. PMID: 32647242 Free PMC article.
-
CD157 in bone marrow mesenchymal stem cells mediates mitochondrial production and transfer to improve neuronal apoptosis and functional recovery after spinal cord injury.Stem Cell Res Ther. 2021 May 17;12(1):289. doi: 10.1186/s13287-021-02305-w. Stem Cell Res Ther. 2021. PMID: 34001228 Free PMC article.
-
The Effects of the Olig Family on the Regulation of Spinal Cord Development and Regeneration.Neurochem Res. 2021 Nov;46(11):2776-2782. doi: 10.1007/s11064-021-03383-1. Epub 2021 Jul 6. Neurochem Res. 2021. PMID: 34228233 Review.
References
-
- Assinck PL, Duncan G, Plemel J, Lee M, Liu J, Bergles D, et al. PDGFRα-positive progenitor cells form myelinating oligodendrocytes and Schwann cells following contusion spinal cord injury. Society for Neuroscience Abstract 2015, Program No. 338.03. Chicago; 2015.
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006; 23: 635–59. May; - PubMed
-
- Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, et al. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol 1997; 148: 453–63.; - PubMed
-
- Bermingham-McDonogh O, Xu YT, Marchionni MA, Scherer SS. Neuregulin expression in PNS neurons: isoforms and regulation by target interactions. Mol Cell Neurosci 1997; 10: 184–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

