Cerebral haemodynamics during experimental intracranial hypertension
- PMID: 26994043
- PMCID: PMC5381462
- DOI: 10.1177/0271678X16639060
Cerebral haemodynamics during experimental intracranial hypertension
Abstract
Intracranial hypertension is a common final pathway in many acute neurological conditions. However, the cerebral haemodynamic response to acute intracranial hypertension is poorly understood. We assessed cerebral haemodynamics (arterial blood pressure, intracranial pressure, laser Doppler flowmetry, basilar artery Doppler flow velocity, and vascular wall tension) in 27 basilar artery-dependent rabbits during experimental (artificial CSF infusion) intracranial hypertension. From baseline (∼9 mmHg; SE 1.5) to moderate intracranial pressure (∼41 mmHg; SE 2.2), mean flow velocity remained unchanged (47 to 45 cm/s; p = 0.38), arterial blood pressure increased (88.8 to 94.2 mmHg; p < 0.01), whereas laser Doppler flowmetry and wall tension decreased (laser Doppler flowmetry 100 to 39.1% p < 0.001; wall tension 19.3 to 9.8 mmHg, p < 0.001). From moderate to high intracranial pressure (∼75 mmHg; SE 3.7), both mean flow velocity and laser Doppler flowmetry decreased (45 to 31.3 cm/s p < 0.001, laser Doppler flowmetry 39.1 to 13.3%, p < 0.001), arterial blood pressure increased still further (94.2 to 114.5 mmHg; p < 0.001), while wall tension was unchanged (9.7 to 9.6 mmHg; p = 0.35).This animal model of acute intracranial hypertension demonstrated two intracranial pressure-dependent cerebroprotective mechanisms: with moderate increases in intracranial pressure, wall tension decreased, and arterial blood pressure increased, while with severe increases in intracranial pressure, an arterial blood pressure increase predominated. Clinical monitoring of such phenomena could help individualise the management of neurocritical patients.
Keywords: Intracranial hypertension; cerebral blood flow; cerebral haemodynamics; intracranial pressure; pressure reactivity; vascular function.
Figures


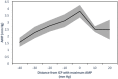
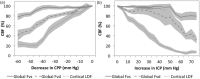

References
-
- Bhatia A, Gupta AK. Neuromonitoring in the intensive care unit. I. Intracranial pressure and cerebral blood flow monitoring. Intensive Care Med 2007; 33: 1263–1271. - PubMed
-
- Beiner JM, Olgivy CS, DuBois AB. Cerebral blood flow changes in response to elevated intracranial pressure in rabbits and bluefish: a comparative study. Comp Biochem Physiol A Physiol 1997; 116: 245–252. - PubMed
-
- Numoto M, Donaghy RM. Effects of local pressure on cortical electrical activity and cortical vessels in the dog. J Neurosurg 1970; 33: 381–387. - PubMed
-
- Zwetnow NN. The influence of an increased intracranial pressure on the lactate, pyruvate, bicarbonate, phosphocreatine, ATP, ADP and AMP concentrations of the cerebral cortex of dogs. Acta Physiol Scand 1970; 79: 158–166. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

