Environmental Metabolomics of the Tomato Plant Surface Provides Insights on Salmonella enterica Colonization
- PMID: 26994076
- PMCID: PMC4959065
- DOI: 10.1128/AEM.00435-16
Environmental Metabolomics of the Tomato Plant Surface Provides Insights on Salmonella enterica Colonization
Abstract
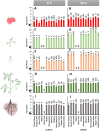
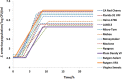
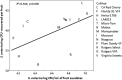
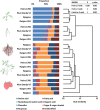
Foodborne illness-causing enteric bacteria are able to colonize plant surfaces without causing infection. We lack an understanding of how epiphytic persistence of enteric bacteria occurs on plants, possibly as an adaptive transit strategy to maximize chances of reentering herbivorous hosts. We used tomato (Solanum lycopersicum) cultivars that have exhibited differential susceptibilities to Salmonella enterica colonization to investigate the influence of plant surface compounds and exudates on enteric bacterial populations. Tomato fruit, shoot, and root exudates collected at different developmental stages supported growth of S. enterica to various degrees in a cultivar- and plant organ-dependent manner. S. enterica growth in fruit exudates of various cultivars correlated with epiphytic growth data (R(2) = 0.504; P = 0.006), providing evidence that plant surface compounds drive bacterial colonization success. Chemical profiling of tomato surface compounds with gas chromatography-time of flight mass spectrometry (GC-TOF-MS) provided valuable information about the metabolic environment on fruit, shoot, and root surfaces. Hierarchical cluster analysis of the data revealed quantitative differences in phytocompounds among cultivars and changes over a developmental course and by plant organ (P < 0.002). Sugars, sugar alcohols, and organic acids were associated with increased S. enterica growth, while fatty acids, including palmitic and oleic acids, were negatively correlated. We demonstrate that the plant surface metabolite landscape has a significant impact on S. enterica growth and colonization efficiency. This environmental metabolomics approach provides an avenue to understand interactions between human pathogens and plants that could lead to strategies to identify or breed crop cultivars for microbiologically safer produce.
Importance: In recent years, fresh produce has emerged as a leading food vehicle for enteric pathogens. Salmonella-contaminated tomatoes represent a recurrent human pathogen-plant commodity pair. We demonstrate that Salmonella can utilize tomato surface compounds and exudates for growth. Surface metabolite profiling revealed that the types and amounts of compounds released to the plant surface differ by cultivar, plant developmental stage, and plant organ. Differences in exudate profiles explain some of the variability in Salmonella colonization susceptibility seen among tomato cultivars. Certain medium- and long-chain fatty acids were associated with restricted Salmonella growth, while sugars, sugar alcohols, and organic acids correlated with larger Salmonella populations. These findings uncover the possibility of selecting crop varieties based on characteristics that impair foodborne pathogen growth for enhanced safety of fresh produce.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- CDC. 2007. Multistate outbreaks of Salmonella infections associated with raw tomatoes eaten in restaurants—United States, 2005-2006. MMWR Morb Mortal Wkly Rep 56:909–911. - PubMed
-
- CDC. 2005. Outbreaks of Salmonella infections associated with eating Roma tomatoes—United States and Canada, 2004. MMWR Morb Mortal Wkly Rep 54:325–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

