Cell migration in the developing rodent olfactory system
- PMID: 26994098
- PMCID: PMC4894936
- DOI: 10.1007/s00018-016-2172-7
Cell migration in the developing rodent olfactory system
Abstract
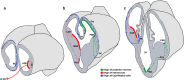
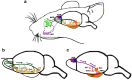
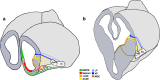
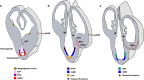
The components of the nervous system are assembled in development by the process of cell migration. Although the principles of cell migration are conserved throughout the brain, different subsystems may predominantly utilize specific migratory mechanisms, or may display unusual features during migration. Examining these subsystems offers not only the potential for insights into the development of the system, but may also help in understanding disorders arising from aberrant cell migration. The olfactory system is an ancient sensory circuit that is essential for the survival and reproduction of a species. The organization of this circuit displays many evolutionarily conserved features in vertebrates, including molecular mechanisms and complex migratory pathways. In this review, we describe the elaborate migrations that populate each component of the olfactory system in rodents and compare them with those described in the well-studied neocortex. Understanding how the components of the olfactory system are assembled will not only shed light on the etiology of olfactory and sexual disorders, but will also offer insights into how conserved migratory mechanisms may have shaped the evolution of the brain.
Keywords: Domains; Evolution; Migration; Neocortex; Olfactory; Vomeronasal.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

