Quantitative Assessment of Eye Phenotypes for Functional Genetic Studies Using Drosophila melanogaster
- PMID: 26994292
- PMCID: PMC4856093
- DOI: 10.1534/g3.116.027060
Quantitative Assessment of Eye Phenotypes for Functional Genetic Studies Using Drosophila melanogaster
Abstract
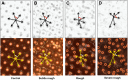
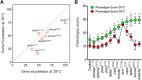
About two-thirds of the vital genes in the Drosophila genome are involved in eye development, making the fly eye an excellent genetic system to study cellular function and development, neurodevelopment/degeneration, and complex diseases such as cancer and diabetes. We developed a novel computational method, implemented as Flynotyper software (http://flynotyper.sourceforge.net), to quantitatively assess the morphological defects in the Drosophila eye resulting from genetic alterations affecting basic cellular and developmental processes. Flynotyper utilizes a series of image processing operations to automatically detect the fly eye and the individual ommatidium, and calculates a phenotypic score as a measure of the disorderliness of ommatidial arrangement in the fly eye. As a proof of principle, we tested our method by analyzing the defects due to eye-specific knockdown of Drosophila orthologs of 12 neurodevelopmental genes to accurately document differential sensitivities of these genes to dosage alteration. We also evaluated eye images from six independent studies assessing the effect of overexpression of repeats, candidates from peptide library screens, and modifiers of neurotoxicity and developmental processes on eye morphology, and show strong concordance with the original assessment. We further demonstrate the utility of this method by analyzing 16 modifiers of sine oculis obtained from two genome-wide deficiency screens of Drosophila and accurately quantifying the effect of its enhancers and suppressors during eye development. Our method will complement existing assays for eye phenotypes, and increase the accuracy of studies that use fly eyes for functional evaluation of genes and genetic interactions.
Keywords: Drosophila melanogaster; human disease models; modifier screens; neurodevelopmental disorders; ommatidia; rough eye.
Copyright © 2016 Iyer et al.
Figures




Similar articles
-
Flynotyper 2.0: an updated tool for rapid quantitative assessment of Drosophila eye phenotypes.G3 (Bethesda). 2024 Nov 6;14(11):jkae212. doi: 10.1093/g3journal/jkae212. G3 (Bethesda). 2024. PMID: 39241113 Free PMC article.
-
Flynotyper 2.0: An updated tool for rapid quantitative assessment of Drosophila eye phenotypes.bioRxiv [Preprint]. 2024 Aug 16:2024.04.07.588481. doi: 10.1101/2024.04.07.588481. bioRxiv. 2024. Update in: G3 (Bethesda). 2024 Nov 6;14(11):jkae212. doi: 10.1093/g3journal/jkae212. PMID: 39185196 Free PMC article. Updated. Preprint.
-
Quantifying Drosophila melanogaster Eye Phenotypes: A Computational Approach Integrating ilastik and Flynotyper.J Vis Exp. 2024 Oct 4;(212). doi: 10.3791/67219. J Vis Exp. 2024. PMID: 39431770
-
Drosophila, genetic screens, and cardiac function.Circ Res. 2011 Sep 16;109(7):794-806. doi: 10.1161/CIRCRESAHA.111.244897. Circ Res. 2011. PMID: 21921272 Free PMC article. Review.
-
FLPing Genes On and Off in Drosophila.Methods Mol Biol. 2017;1642:195-209. doi: 10.1007/978-1-4939-7169-5_13. Methods Mol Biol. 2017. PMID: 28815502 Free PMC article. Review.
Cited by
-
Herzog is not required for mushroom body development or courtship learning & memory but is required for eye development in Drosophila melanogaster.MicroPubl Biol. 2023 Jan 30;2023:10.17912/micropub.biology.000720. doi: 10.17912/micropub.biology.000720. eCollection 2023. MicroPubl Biol. 2023. PMID: 36798589 Free PMC article.
-
Fly for ALS: Drosophila modeling on the route to amyotrophic lateral sclerosis modifiers.Cell Mol Life Sci. 2021 Sep;78(17-18):6143-6160. doi: 10.1007/s00018-021-03905-8. Epub 2021 Jul 28. Cell Mol Life Sci. 2021. PMID: 34322715 Free PMC article. Review.
-
Probing the conserved roles of cut in the development and function of optically different insect compound eyes.Front Cell Dev Biol. 2023 Mar 31;11:1104620. doi: 10.3389/fcell.2023.1104620. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37065850 Free PMC article.
-
Flynotyper 2.0: an updated tool for rapid quantitative assessment of Drosophila eye phenotypes.G3 (Bethesda). 2024 Nov 6;14(11):jkae212. doi: 10.1093/g3journal/jkae212. G3 (Bethesda). 2024. PMID: 39241113 Free PMC article.
-
Neuronal Glycogen Breakdown Mitigates Tauopathy via Pentose Phosphate Pathway-Mediated Oxidative Stress Reduction.Res Sq [Preprint]. 2023 Nov 8:rs.3.rs-3526342. doi: 10.21203/rs.3.rs-3526342/v1. Res Sq. 2023. Update in: Nat Metab. 2025 Jul;7(7):1375-1391. doi: 10.1038/s42255-025-01314-w. PMID: 37986935 Free PMC article. Updated. Preprint.
References
-
- Arulmozhi K., Perumal S. A., Sanooj P., Nallaperumal K., 2012. Application of Top Hat transform technique on Indian license plate image localization, pp. 708–711 in 2012 Ieee International Conference on Computational Intelligence and Computing Research (Iccic).
-
- Basler K., Christen B., Hafen E., 1991. Ligand-independent activation of the sevenless receptor tyrosine kinase changes the fate of cells in the developing Drosophila eye. Cell 64(6): 1069–1081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
