Human RAG mutations: biochemistry and clinical implications
- PMID: 26996199
- PMCID: PMC5757527
- DOI: 10.1038/nri.2016.28
Human RAG mutations: biochemistry and clinical implications
Abstract
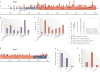
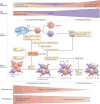
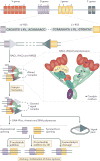
The recombination-activating gene 1 (RAG1) and RAG2 proteins initiate the V(D)J recombination process, which ultimately enables the generation of T cells and B cells with a diversified repertoire of antigen-specific receptors. Mutations of the RAG genes in humans are associated with a broad spectrum of clinical phenotypes, ranging from severe combined immunodeficiency to autoimmunity. Recently, novel insights into the phenotypic diversity of this disease have been provided by resolving the crystal structure of the RAG complex, by developing novel assays to test recombination activity of the mutant RAG proteins and by characterizing the molecular and cellular basis of immune dysregulation in patients with RAG deficiency.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. - PubMed
-
- Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. - PubMed
-
- Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. - PubMed
-
- Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. - PubMed
-
- Schwarz K, et al. RAG mutations in human B cell-negative SCID. Science. 1996;274:97–99. This is the first study to report that RAG mutations in humans cause SCID. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

