A Host-Produced Autoinducer-2 Mimic Activates Bacterial Quorum Sensing
- PMID: 26996306
- PMCID: PMC4869860
- DOI: 10.1016/j.chom.2016.02.020
A Host-Produced Autoinducer-2 Mimic Activates Bacterial Quorum Sensing
Abstract
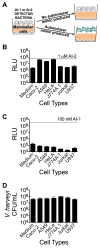
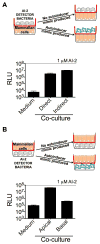
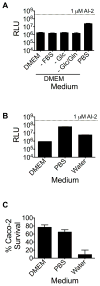
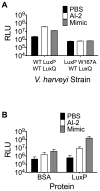
Host-microbial symbioses are vital to health; nonetheless, little is known about the role crosskingdom signaling plays in these relationships. In a process called quorum sensing, bacteria communicate with one another using extracellular signal molecules called autoinducers. One autoinducer, AI-2, is proposed to promote interspecies bacterial communication, including in the mammalian gut. We show that mammalian epithelia produce an AI-2 mimic activity in response to bacteria or tight-junction disruption. This AI-2 mimic is detected by the bacterial AI-2 receptor, LuxP/LsrB, and can activate quorum-sensing-controlled gene expression, including in the enteric pathogen Salmonella typhimurium. AI-2 mimic activity is induced when epithelia are directly or indirectly exposed to bacteria, suggesting that a secreted bacterial component(s) stimulates its production. Mutagenesis revealed genes required for bacteria to both detect and stimulate production of the AI-2 mimic. These findings uncover a potential role for the mammalian AI-2 mimic in fostering crosskingdom signaling and host-bacterial symbioses.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
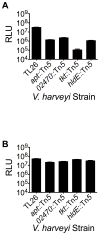
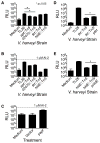
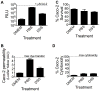
Comment in
-
Looks Who's Talking Now.Cell Host Microbe. 2016 Apr 13;19(4):429-30. doi: 10.1016/j.chom.2016.03.013. Cell Host Microbe. 2016. PMID: 27078063
Similar articles
-
Saccharomyces cerevisiae Requires CFF1 To Produce 4-Hydroxy-5-Methylfuran-3(2H)-One, a Mimic of the Bacterial Quorum-Sensing Autoinducer AI-2.mBio. 2021 Mar 9;12(2):e03303-20. doi: 10.1128/mBio.03303-20. mBio. 2021. PMID: 33688008 Free PMC article.
-
Quorum sensing in thermophiles: prevalence of autoinducer-2 system.BMC Microbiol. 2018 Jun 28;18(1):62. doi: 10.1186/s12866-018-1204-x. BMC Microbiol. 2018. PMID: 29954335 Free PMC article.
-
Identification of novel autoinducer-2 receptors in Clostridia reveals plasticity in the binding site of the LsrB receptor family.J Biol Chem. 2019 Mar 22;294(12):4450-4463. doi: 10.1074/jbc.RA118.006938. Epub 2019 Jan 29. J Biol Chem. 2019. PMID: 30696769 Free PMC article.
-
Molecules Autoinducer 2 and cjA and Their Impact on Gene Expression in Campylobacter jejuni.J Mol Microbiol Biotechnol. 2018;28(5):207-215. doi: 10.1159/000495411. Epub 2019 Jan 29. J Mol Microbiol Biotechnol. 2018. PMID: 30695777 Review.
-
Bacterial interspecies quorum sensing in the mammalian gut microbiota.C R Biol. 2018 May-Jun;341(5):297-299. doi: 10.1016/j.crvi.2018.03.006. Epub 2018 Apr 7. C R Biol. 2018. PMID: 29631889 Review.
Cited by
-
The New Antibacterial Properties of the Plants: Quo vadis Studies of Anti-virulence Phytochemicals?Front Microbiol. 2021 May 7;12:667126. doi: 10.3389/fmicb.2021.667126. eCollection 2021. Front Microbiol. 2021. PMID: 34025622 Free PMC article. Review.
-
Altruism and Phenoptosis as Programs Supported by Evolution.Biochemistry (Mosc). 2021 Dec;86(12):1540-1552. doi: 10.1134/S0006297921120038. Biochemistry (Mosc). 2021. PMID: 34937533 Free PMC article.
-
Regular Wounding in a Natural System: Bacteria Associated With Reproductive Organs of Bedbugs and Their Quorum Sensing Abilities.Front Immunol. 2017 Dec 18;8:1855. doi: 10.3389/fimmu.2017.01855. eCollection 2017. Front Immunol. 2017. PMID: 29326722 Free PMC article.
-
Molecular trafficking between bacteria determines the shape of gut microbial community.Gut Microbes. 2021 Jan-Dec;13(1):1959841. doi: 10.1080/19490976.2021.1959841. Gut Microbes. 2021. PMID: 34455923 Free PMC article. Review.
-
Interplay between chemotaxis, quorum sensing, and metabolism regulates Escherichia coli-Salmonella Typhimurium interactions in vivo.PLoS Pathog. 2025 May 2;21(5):e1013156. doi: 10.1371/journal.ppat.1013156. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40315408 Free PMC article.
References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. - PubMed
-
- Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–86. - PubMed
-
- Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol. 1994;13:273–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

