Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home
- PMID: 26996542
- PMCID: PMC5111773
- DOI: 10.1111/dom.12663
Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home
Abstract
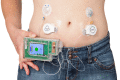
Aims: To assess the performance and safety of an integrated bihormonal artificial pancreas system consisting of one wearable device and two wireless glucose sensor transmitters during short-term daily use at home.
Methods: Adult patients with type 1 diabetes using an insulin pump were invited to enrol in this randomized crossover study. Treatment with the artificial pancreas started with a day and night in the clinical research centre, followed by 3 days at home. The control period consisted of 4 days of insulin pump therapy at home with blinded continuous glucose monitoring for data collection. Days 2-4 were predefined as the analysis period, with median glucose as the primary outcome.
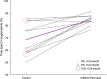
Results: A total of 10 patients completed the study. The median [interquartile range (IQR)] glucose level was similar for the two treatments [7.3 (7.0-7.6) mmol/l for the artificial pancreas vs. 7.7 (7.0-9.0) mmol/l for the control; p = 0.123]. The median (IQR) percentage of time spent in euglycaemia (3.9-10 mmol/l) was longer during use of the artificial pancreas [84.7 (82.2-87.8)% for the artificial pancreas vs. 68.5 (57.9-83.6)% for the control; p = 0.007]. Time in hypoglycaemia was 1.3 (0.2-3.2)% for the artificial pancreas and 2.4 (0.4-10.3)% for the control treatment (p = 0.139). Separate analysis of daytime and night-time showed that the improvements were mainly achieved during the night.
Conclusions: The results of this pilot study suggest that our integrated artificial pancreas provides better glucose control than insulin pump therapy in patients with type 1 diabetes at home and that the treatment is safe.
Keywords: clinical trial; glucagon; glycaemic control; insulin therapy; type 1 diabetes.
© 2016 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures

References
-
- Nimri R, Muller I, Atlas E et al. MD‐logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care 2014; 37: 3025–3032. - PubMed
-
- Kropff J, Del Favero S, Place J et al. 2 month evening and night closed‐loop glucose control in patients with type 1 diabetes under free‐living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol 2015; 3: 939–947. - PubMed
-
- Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa‐Lhoret R. Comparison of dual‐hormone artificial pancreas, single‐hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open‐label randomised controlled crossover trial. Lancet Diabetes Endocrinol 2015; 3: 17–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

