Periodic forces trigger knot untying during translocation of knotted proteins
- PMID: 26996878
- PMCID: PMC4800218
- DOI: 10.1038/srep21702
Periodic forces trigger knot untying during translocation of knotted proteins
Abstract
Proteins need to be unfolded when translocated through the pores in mitochondrial and other cellular membranes. Knotted proteins, however, might get stuck during this process, jamming the pore, since the diameter of the pore is smaller than the size of maximally tightened knot. The jamming probability dramatically increases as the magnitude of the driving force exceeds a critical value, Fc. In this numerical study, we show that for deep knots Fc lies below the force range over which molecular import motors operate, which suggest that in these cases the knots will tighten and block the pores. Next, we show how such topological traps might be prevented by using a pulling protocol of a repetitive, on-off character. Such a repetitive pulling is biologically relevant, since the mitochondrial import motor, like other molecular motors transforms chemical energy into directed motions via nucleotide-hydrolysis-mediated conformational changes, which are cyclic in character.
Figures
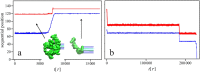
 trajectory is shown in panel (a), however no further changes in the knot position were observed beyond
trajectory is shown in panel (a), however no further changes in the knot position were observed beyond  .
.
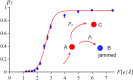
 ,
,  and
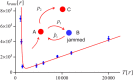
and  . The inset shows schematically the kinetic partitioning between the translocation
. The inset shows schematically the kinetic partitioning between the translocation  and knot tightening
and knot tightening  . Error bars mark 68% Wilson confidence intervals.
. Error bars mark 68% Wilson confidence intervals.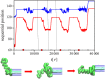
 and
and  , estimated as described in the text. The error bars mark the standard deviation from the mean.
, estimated as described in the text. The error bars mark the standard deviation from the mean.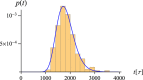
 with
with  and
and  .
.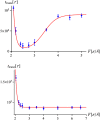
 . The red curve in the top panel represents the formula
. The red curve in the top panel represents the formula  , as given by Eq. (4) . The values of the parameters are
, as given by Eq. (4) . The values of the parameters are  ,
,  ,
,  , whereas
, whereas  is given by Eq. (1) with
is given by Eq. (1) with  and
and  . In the bottom panel, the red curve is given by
. In the bottom panel, the red curve is given by  , since the forces involved are much larger than Fc for this protein (cf. Table 1). The remaining parameters are
, since the forces involved are much larger than Fc for this protein (cf. Table 1). The remaining parameters are  ,
,  ,
,  and
and  . The error bars mark the standard deviation from the mean.
. The error bars mark the standard deviation from the mean.Similar articles
-
Revealing Topological Barriers against Knot Untying in Thermal and Mechanical Protein Unfolding by Molecular Dynamics Simulations.Biomolecules. 2021 Nov 13;11(11):1688. doi: 10.3390/biom11111688. Biomolecules. 2021. PMID: 34827686 Free PMC article.
-
Tight knots in proteins: can they block the mitochondrial pores?Biochem Soc Trans. 2013 Apr;41(2):620-4. doi: 10.1042/BST20120261. Biochem Soc Trans. 2013. PMID: 23514165 Review.
-
Mechanically tightening a protein slipknot into a trefoil knot.J Am Chem Soc. 2014 Aug 27;136(34):11946-55. doi: 10.1021/ja503997h. Epub 2014 Aug 13. J Am Chem Soc. 2014. PMID: 25092607
-
Stochastic model of translocation of knotted proteins.Phys Rev E. 2022 Nov;106(5-1):054406. doi: 10.1103/PhysRevE.106.054406. Phys Rev E. 2022. PMID: 36559434
-
The mitochondrial protein import motor.Biol Chem. 2000 Sep-Oct;381(9-10):943-9. doi: 10.1515/BC.2000.115. Biol Chem. 2000. PMID: 11076025 Review.
Cited by
-
Revealing Topological Barriers against Knot Untying in Thermal and Mechanical Protein Unfolding by Molecular Dynamics Simulations.Biomolecules. 2021 Nov 13;11(11):1688. doi: 10.3390/biom11111688. Biomolecules. 2021. PMID: 34827686 Free PMC article.
-
The AAA+ protease ClpXP can easily degrade a 31 and a 52-knotted protein.Sci Rep. 2019 Feb 20;9(1):2421. doi: 10.1038/s41598-018-38173-3. Sci Rep. 2019. PMID: 30787316 Free PMC article.
-
Insights into protein sequencing with an α-Hemolysin nanopore by atomistic simulations.Sci Rep. 2019 Apr 23;9(1):6440. doi: 10.1038/s41598-019-42867-7. Sci Rep. 2019. PMID: 31015503 Free PMC article.
-
Observations of metastable states of the free swelling knots and directional motion of tensioned knots in vibrated bead chains.Eur Phys J E Soft Matter. 2019 Jun 25;42(6):79. doi: 10.1140/epje/i2019-11841-8. Eur Phys J E Soft Matter. 2019. PMID: 31227934
-
Polymer translocation through nano-pores in vibrating thin membranes.Sci Rep. 2016 Dec 9;6:38558. doi: 10.1038/srep38558. Sci Rep. 2016. PMID: 27934936 Free PMC article.
References
-
- Frank-Kamenetskii M. D., Lukashin A. V. & Vologodskii A. V. Statistical mechanics and topology of polymer chains. Nature 258, 398–402 (1975). - PubMed
-
- Sumners D. & Whittington S. G. Knots in self-avoiding walks. J. Phys. A: Math. Gen. 21, 1689 (1988).
-
- Bates A. D., Maxwell A. DNA Topology (Oxford University Press, 2005).
-
- Taylor W. R. & Lin K. Protein knots: a tangled problem. Nature 421, 25–25 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

