Invited review: Activation of G proteins by GTP and the mechanism of Gα-catalyzed GTP hydrolysis
- PMID: 26996924
- PMCID: PMC5319639
- DOI: 10.1002/bip.22836
Invited review: Activation of G proteins by GTP and the mechanism of Gα-catalyzed GTP hydrolysis
Abstract
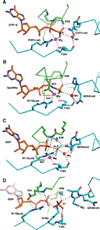
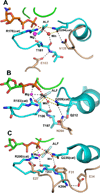
This review addresses the regulatory consequences of the binding of GTP to the alpha subunits (Gα) of heterotrimeric G proteins, the reaction mechanism of GTP hydrolysis catalyzed by Gα and the means by which GTPase activating proteins (GAPs) stimulate the GTPase activity of Gα. The high energy of GTP binding is used to restrain and stabilize the conformation of the Gα switch segments, particularly switch II, to afford stable complementary to the surfaces of Gα effectors, while excluding interaction with Gβγ, the regulatory binding partner of GDP-bound Gα. Upon GTP hydrolysis, the energy of these conformational restraints is dissipated and the two switch segments, particularly switch II, become flexible and are able to adopt a conformation suitable for tight binding to Gβγ. Catalytic site pre-organization presents a significant activation energy barrier to Gα GTPase activity. The glutamine residue near the N-terminus of switch II (Glncat ) must adopt a conformation in which it orients and stabilizes the γ phosphate and the water nucleophile for an in-line attack. The transition state is probably loose with dissociative character; phosphoryl transfer may be concerted. The catalytic arginine in switch I (Argcat ), together with amide hydrogen bonds from the phosphate binding loop, stabilize charge at the β-γ bridge oxygen of the leaving group. GAPs that harbor "regulator of protein signaling" (RGS) domains, or structurally unrelated domains within G protein effectors that function as GAPs, accelerate catalysis by stabilizing the pre-transition state for Gα-catalyzed GTP hydrolysis, primarily by restraining Argcat and Glncat to their catalytic conformations. © 2016 Wiley Periodicals, Inc. Biopolymers 105: 449-462, 2016.
Keywords: GTPase activating proteins; Heterotrimeric G proteins; Reaction mechanism; Signal Transduction; X-ray crystallography.
© 2016 Wiley Periodicals, Inc.
Figures
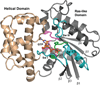
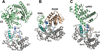
References
-
- Cassel D, Selinger Z. Biochim Biophys Acta. 1976;452:538–551. - PubMed
-
- Pfeuffer T, Helmreich EJ. J Biol Chem. 1975;250:867–876. - PubMed
-
- Ross EM, Howlett AC, Ferguson KM, Gilman AG. J Biol Chem. 1978;253:6401–6412. - PubMed
-
- Pfeuffer T. J Biol Chem. 1977;252:7224–7234. - PubMed
-
- Gilman AG. Anual Reveiws in Biochemistry. 1987;56:615–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

