First Introduction of NiSe2 to Anode Material for Sodium-Ion Batteries: A Hybrid of Graphene-Wrapped NiSe2/C Porous Nanofiber
- PMID: 26997350
- PMCID: PMC4800391
- DOI: 10.1038/srep23338
First Introduction of NiSe2 to Anode Material for Sodium-Ion Batteries: A Hybrid of Graphene-Wrapped NiSe2/C Porous Nanofiber
Abstract
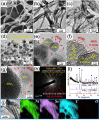
The first-ever study of nickel selenide materials as efficient anode materials for Na-ion rechargeable batteries is conducted using the electrospinning process. NiSe2-reduced graphene oxide (rGO)-C composite nanofibers are successfully prepared via electrospinning and a subsequent selenization process. The electrospun nanofibers giving rise to these porous-structured composite nanofibers with optimum amount of amorphous C are obtained from the polystyrene to polyacrylonitrile ratio of 1/4. These composite nanofibers also consist of uniformly distributed single-crystalline NiSe2 nanocrystals that have a mean size of 27 nm. In contrast, the densely structured bare NiSe2 nanofibers formed via selenization of the pure NiO nanofibers consist of large crystallites. The initial discharge capacities of the NiSe2-rGO-C composite and bare NiSe2 nanofibers at a current density of 200 mA g(-1) are 717 and 755 mA h g(-1), respectively. However, the respective 100(th)-cycle discharge capacities of the former and latter are 468 and 35 mA h g(-1). Electrochemical impedance spectroscopy measurements reveal the structural stability of the composite nanofibers during repeated Na-ion insertion and extraction processes. The excellent Na-ion storage properties of these nanofibers are attributed to this structural stability.
Figures





References
-
- Li L. et al.. A flexible quasi‐solid‐state asymmetric electrochemical capacitor based on hierarchical porous V2O5 nanosheets on carbon nanofibers. Adv. Energy Mater. 5, 1500753 (2015).
-
- Xu W. et al.. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
-
- You Y., Wu X. L., Yin Y. X. & Guo Y. G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 7, 1643–1647 (2014).
-
- Xu Y. et al.. Confined sulfur in microporous carbon renders superior cycling stability in Li/S batteries. Adv. Funct. Mater. 25, 4312–4320 (2015).
-
- Wei T., Gong Y., Zhao X. & Huang K. An all‐ceramic solid‐state rechargeable Na+ battery operated at intermediate temperatures. Adv. Funct. Mater. 24, 5380–5384 (2014).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

