Efficacy and safety of ursodeoxycholic acid composite on fatigued patients with elevated liver function and/or fatty liver: a multi-centre, randomised, double-blinded, placebo-controlled trial
- PMID: 26997458
- PMCID: PMC5071730
- DOI: 10.1111/ijcp.12790
Efficacy and safety of ursodeoxycholic acid composite on fatigued patients with elevated liver function and/or fatty liver: a multi-centre, randomised, double-blinded, placebo-controlled trial
Erratum in
-
Corrigendum.Int J Clin Pract. 2017 Feb;71(2):e12930. doi: 10.1111/ijcp.12930. Int J Clin Pract. 2017. PMID: 28238230 Free PMC article. No abstract available.
Abstract
Aim: The aim of this study was to assess the effects of ursodeoxycholic acid composite (URSA-S) on fatigue in patients with elevated liver function tests and/or fatty liver disease.
Methods: In this multi-centre randomised double-blinded placebo-controlled trial, 168 adults who were diagnosed with fatigue based on our criteria and had elevated liver function tests (but not > 5 times the normal level) and/or fatty liver on ultrasonography, were randomised to either the placebo or URSA-S administration group. The rate of improvement of checklist individual strength (CIS) using a cut-off of 76 points at the end of the study (8 weeks), the change in fatigue scale [CIS score and visual analogue scale (VAS)] were evaluated. The adverse effects of URSA-S were also recorded.
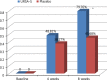
Results: The rate of CIS improvement at the end-point was 79.76% and 45.68% in the therapy and placebo groups, respectively (p < 0.05). The fatigue recovery rate of the CIS score and VAS were higher in the therapy (-25.44 ± 18.57, -27.84 ± 2.70) than in the placebo group (-16.59 ± 17.29, -19.46 ± 2.81) (p < 0.05). The difference in fatigue recovery rate between the therapy and placebo groups was significant after 8 weeks. When analysed separately in patients with abnormal liver function tests and fatty liver disease, the fatigue recovery rate of the CIS score and VAS at 8 weeks was higher in the therapy than in the placebo group (p < 0.05). The frequency of adverse events in the therapy group was not significantly higher than that in the placebo group.
Conclusion: URSA-S is effective for alleviating fatigue in patients with liver dysfunction and/or fatty liver. The adverse effects of URSA-S are not significant. This study is registered at https://clinicaltrials.gov/ct2/show/NCT02415777.
© 2016 The Authors. International Journal of Clinical Practice Published by John Wiley & Sons Ltd.
Figures
References
-
- Krupp LB, Pollina DA. Mechanisms and management of fatigue in progressive neurological disorders. Curr Opin Neurol 1996; 9: 456–60. - PubMed
-
- Berrios GE. Feelings of fatigue and psychopathology: a conceptual history. Compr Psychiatry 1990; 31: 140–51. - PubMed
-
- Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology 2011; 50: 1009–18. - PubMed
-
- Copaci I, Micu L, Iliescu L, Voiculescu M. New therapeutical indications of ursodeoxycholic acid. Rom J Gastroenterol 2005; 14: 259–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical