Neuronal Firing Rate Homeostasis Is Inhibited by Sleep and Promoted by Wake
- PMID: 26997481
- PMCID: PMC4809041
- DOI: 10.1016/j.cell.2016.01.046
Neuronal Firing Rate Homeostasis Is Inhibited by Sleep and Promoted by Wake
Abstract
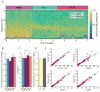
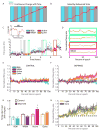
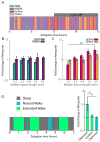
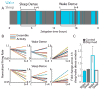
Homeostatic mechanisms stabilize neural circuit function by keeping firing rates within a set-point range, but whether this process is gated by brain state is unknown. Here, we monitored firing rate homeostasis in individual visual cortical neurons in freely behaving rats as they cycled between sleep and wake states. When neuronal firing rates were perturbed by visual deprivation, they gradually returned to a precise, cell-autonomous set point during periods of active wake, with lengthening of the wake period enhancing firing rate rebound. Unexpectedly, this resetting of neuronal firing was suppressed during sleep. This raises the possibility that memory consolidation or other sleep-dependent processes are vulnerable to interference from homeostatic plasticity mechanisms. PAPERCLIP.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Cortical physiology: Waking up homeostasis.Nat Rev Neurosci. 2016 May;17(5):261. doi: 10.1038/nrn.2016.45. Epub 2016 Apr 7. Nat Rev Neurosci. 2016. PMID: 27052384 No abstract available.
References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

