Modeling Intercellular Communication as a Survival Strategy of Cancer Cells: An In Silico Approach on a Flexible Bioinformatics Framework
- PMID: 26997867
- PMCID: PMC4790585
- DOI: 10.4137/BBI.S38075
Modeling Intercellular Communication as a Survival Strategy of Cancer Cells: An In Silico Approach on a Flexible Bioinformatics Framework
Abstract
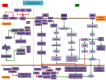
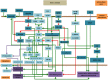
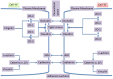
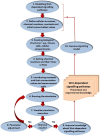
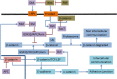
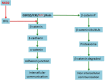
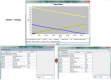
Intercellular communication is very important for cell development and allows a group of cells to survive as a population. Cancer cells have a similar behavior, presenting the same mechanisms and characteristics of tissue formation. In this article, we model and simulate the formation of different communication channels that allow an interaction between two cells. This is a first step in order to simulate in the future processes that occur in healthy tissue when normal cells surround a cancer cell and to interrupt the communication, thus preventing the spread of malignancy into these cells. The purpose of this study is to propose key molecules, which can be targeted to allow us to break the communication between cancer cells and surrounding normal cells. The simulation is carried out using a flexible bioinformatics platform that we developed, which is itself based on the metaphor chemistry-based model.
Keywords: bioinformatics framework; cancer cells; in silico experiments; intracellular communication.
Figures








References
-
- Niklas KJ, Newman SA. The origins of multicellular organisms. Evol Dev. 2013;15:41–52. - PubMed
-
- Brücher BLDM, Jamall IS. Cell-cell communication in the tumor microenvironment, carcinogenesis, and anticancer treatment. Cell Physiol Biochem. 2014;34:213–43. - PubMed
-
- Vinken M. Non-channel functions of connexins in cell growth and cell death. Biochim Biophys Acta. 2012;1818(8):2002–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

