Proteomic analysis of the biomass hydrolytic potentials of Penicillium oxalicum lignocellulolytic enzyme system
- PMID: 26997974
- PMCID: PMC4797192
- DOI: 10.1186/s13068-016-0477-2
Proteomic analysis of the biomass hydrolytic potentials of Penicillium oxalicum lignocellulolytic enzyme system
Abstract
Background: The mining of high-performance enzyme systems is necessary to develop industrial lignocellulose bioconversion. Large amounts of cellulases and hemicellulases can be produced by Penicillium oxalicum. Hence, the enzyme system of this hypercellulolytic fungus should be elucidated to help design optimum enzyme systems for effective biomass hydrolysis.
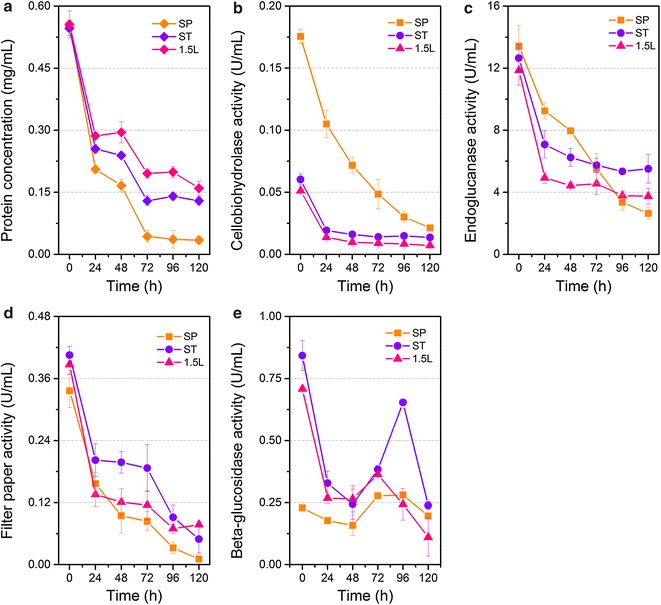
Results: The cellulolytic and xylanolytic activities of an SP enzyme system prepared from P. oxalicum JU-A10 were comparatively analyzed. Results indicated that the fungus possesses a complete cellulolytic-xylanolytic enzyme system. The cellobiohydrolase- and xylanase-specific activities of this system were higher than those of two other enzyme systems, i.e., ST from Trichoderma reesei SN1 and another commercial preparation Celluclast 1.5L. Delignified corncob residue (DCCR) could be hydrolyzed by SP to a greater extent than corncob residue (CCR). Beta-glucosidase (BG) supplemented in SP increased the ability of the system to hydrolyze DCCR and CCR, and resulted in a 64 % decrease in enzyme dosage with the same glucose yield. The behaviors of the enzyme components in the hydrolysis of CCR were further investigated by monitoring individual enzyme dynamics. The total protein concentrations and cellobiohydrolase (CBH), endoglucanase (EG), and filter paper activities in the supernatants significantly decreased during saccharification. These findings were more evident in SP than in the other enzyme systems. The comparative proteomic analysis of the enzyme systems revealed that both SP and ST were rich in carbohydrate-degrading enzymes and multiple non-hydrolytic proteins. A larger number of carbohydrate-binding modules 1 (CBM1) were also identified in SP than in ST. This difference might be linked to the greater adsorption to substrates and lower hydrolysis efficiency of SP enzymes than ST during lignocellulose saccharification, because CBM1 not only targets enzymes to insoluble cellulose but also leads to non-productive adsorption to lignin.
Conclusions: Penicillium oxalicum can be applied to the biorefinery of lignocellulosic biomass. Its ability to degrade lignocellulosic substrates could be further improved by modifying its enzyme system on the basis of enzyme activity measurement and proteomic analysis. The proposed strategy may also be applied to other lignocellulolytic enzyme systems to enhance their hydrolytic performances rationally.
Keywords: Adsorption; Cellulase; Enzymatic hydrolysis; Penicillium oxalicum; Proteomic; Trichoderma reesei.
Figures








References
-
- Rahikainen JL, Martin-Sampedro R, Heikkinen H, Rovio S, Marjamaa K, Tamminen T, et al. Inhibitory effect of lignin during cellulose bioconversion: the effect of lignin chemistry on non-productive enzyme adsorption. Bioresour Technol. 2013;133:270–278. doi: 10.1016/j.biortech.2013.01.075. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

