Efficacy and safety of testosterone replacement therapy in men with hypogonadism: A meta-analysis study of placebo-controlled trials
- PMID: 26998003
- PMCID: PMC4774360
- DOI: 10.3892/etm.2015.2957
Efficacy and safety of testosterone replacement therapy in men with hypogonadism: A meta-analysis study of placebo-controlled trials
Abstract
The purpose of the present meta-analysis was to evaluate the efficacy and safety of testosterone replacement therapy in men with hypogonadism. A search was conducted for appropriate randomized controlled trials and the data from 16 trials were pooled. The intended primary outcome of the present study was to determine the efficacy and safety of testosterone replacement therapy. The current data demonstrated that scores for Aging Male Symptoms (AMS) were significantly reduced following testosterone replacement therapy, with a mean decrease in AMS score of 1.52 [95% confidence interval (CI), 0.72 to 2.32; P=0.0002]. Testosterone replacement therapy increased lean body mass [mean difference (MD), 1.22; 95% CI, 0.33 to 2.11; P=0.007], reduced fat mass in a non-significantly manner (MD, -0.85; 95% CI, -1.74 to 0.04; P=0.06) and significantly reduced total cholesterol (MD, -0.16; 95% CI, -0.29 to -0.03; P=0.01). No significant differences were identified in body weight (MD, 0.09; 95% CI, -1.13 to 1.31; P=0.89), body mass index (MD, 0.10; 95% CI, -0.62 to 0.82; P=0.78) or bone mineral density (MD, -0.01; 95% CI, -0.03 to 0.02; P=0.60). Average prostate volume increased (MD, 1.58; 95% CI, 0.6 to 2.56; P=0.002) following testosterone replacement therapy, but the levels of prostate-specific antigen (PSA) (MD, 0.10; 95% CI, -0.03 to 0.22; P=0.14) and the International Prostate Symptom Scores (MD, 0.01; 95% CI, -0.37 to 0.39; P=0.96) did not change. In conclusion, testosterone replacement therapy improves quality of life, increases lean body mass, significantly decreases total cholesterol, and is well-tolerated and safe for men with hypogonadism who are exhibiting PSA levels of <4 ng/ml.
Keywords: endocrinology; hypogonadal men; testosterone replacement therapy.
Figures
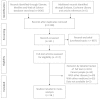

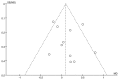
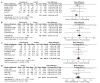

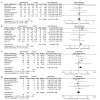
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
