Ibandronate: The loading dose concept in the treatment of metastatic bone pain
- PMID: 26998420
- PMCID: PMC4782017
- DOI: 10.1016/j.jbo.2015.11.001
Ibandronate: The loading dose concept in the treatment of metastatic bone pain
Abstract
Background/aim: Severe bone pain is experienced by 60-80% of patients with metastatic bone disease, and has a profound impact on quality of life. Therefore, effective pain relief is an important goal in managing metastatic bone disease. Orthopedic surgeons are often challenged with patients presenting with newly diagnosed bone metastases and severe and disabling bone pain. It is important to provide fast and sufficient analgesia. Clinical trials have demonstrated that bisphosphonates reduce effectively and sustained bone pain by approved standard dosage over time. Open label prospective trials have shown that short time high dose i.v. Ibandronate is effective in rapid pain relief in different primary tumors.
Patients and methods: In 33 patients with metastatic bone pain from newly diagnosed skeletal metastases we utilized the loading-dose concept for intravenous ibandronate (6 mg infused over 1 h on 3 consecutive days).
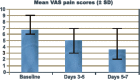
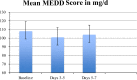
Results: In 33 patients loading-dose ibandronate therapy significantly reduced bone pain within the first 5-7 days (VAS day 0: 6-8 vs. day 7: 3-4). Only 3 patients showed no response concerning a distinct pain reduction within the first days of therapy. There was no increase in pain medication.
Conclusion: This clinical observational study in selected patients with severe metastatic bone pain undergoing an intensive high dosed ibandronate-therapy for a short period demonstrated that loading-dose ibandronate (6 mg i.v., 3 consecutive days) resulted in a reduction of pain within days.
Keywords: Bisphosphonate; Bone metastases; Ibandronate; Pain relief.
Figures
Similar articles
-
Ibandronate in metastatic bone pain.Semin Oncol. 2004 Oct;31(5 Suppl 10):67-72. doi: 10.1053/j.seminoncol.2004.07.026. Semin Oncol. 2004. PMID: 15490379 Review.
-
Efficacy and safety of ibandronate in the treatment of opioid-resistant bone pain associated with metastatic bone disease: a pilot study.J Clin Oncol. 2004 Sep 1;22(17):3587-92. doi: 10.1200/JCO.2004.07.054. J Clin Oncol. 2004. PMID: 15337809
-
Ibandronate: its role in metastatic breast cancer.Oncologist. 2006;11 Suppl 1:27-33. doi: 10.1634/theoncologist.11-90001-27. Oncologist. 2006. PMID: 16971737 Review.
-
Review of ibandronate in the treatment of metastatic bone disease: experience from phase III trials.Clin Ther. 2004 Dec;26(12):1947-59. doi: 10.1016/j.clinthera.2004.12.010. Clin Ther. 2004. PMID: 15823760 Review.
-
A review of bone pain relief with ibandronate and other bisphosphonates in disorders of increased bone turnover.Clin Exp Rheumatol. 2007 Sep-Oct;25(5):766-74. Clin Exp Rheumatol. 2007. PMID: 18078631 Review.
Cited by
-
Bone microenvironment signaling of cancer stem cells as a therapeutic target in metastatic prostate cancer.Cell Biol Toxicol. 2020 Apr;36(2):115-130. doi: 10.1007/s10565-019-09483-7. Epub 2019 Jun 27. Cell Biol Toxicol. 2020. PMID: 31250347 Free PMC article. Review.
References
-
- Body J.J., Mancini I. Bisphosphonates for cancer patients: why, how, and when? Support. Care Cancer. 2002;10:399–407. - PubMed
-
- Mancini I., Dumon J.C., Body J.J. Efficacy and safety of ibandronate in the treatment of opioid-resistant bone pain associated with metastatic bone disease: a pilot study. J. Clin. Oncol. 2004;22:3587–3592. - PubMed
-
- Janjan N. Bone metastases: approaches to management. Semin. Oncol. 2001;28:28–34. - PubMed
-
- Halvorson K.G., Sevcik M.A., Ghilardi J.R., Sullivan L.J., Koewler N.J., Bauss F., Mantyh P.W. Intravenous ibandronate rapidly reduces pain, neurochemical indices of central sensitization, tumor burden, and skeletal destruction in a mouse model of bone cancer. J. Pain. Symptom Manag. 2008;36:289–303. - PMC - PubMed
-
- Mercadante S. Breakthrough pain: on the road again. Eur. J. Pain. 2009;13:329–330. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources