Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy
- PMID: 26998515
- PMCID: PMC4794275
- DOI: 10.1038/npjbcancer.2015.25
Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy
Abstract
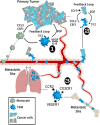
Deleterious inflammation is a primary feature of breast cancer. Accumulating evidence demonstrates that macrophages, the most abundant leukocyte population in mammary tumors, have a critical role at each stage of cancer progression. Such tumor-associated macrophages facilitate neoplastic transformation, tumor immune evasion and the subsequent metastatic cascade. Herein, we discuss the dynamic process whereby molecular and cellular features of the tumor microenvironment act to license tissue-repair mechanisms of macrophages, fostering angiogenesis, metastasis and the support of cancer stem cells. We illustrate how tumors induce, then exploit trophic macrophages to subvert innate and adaptive immune responses capable of destroying malignant cells. Finally, we discuss compelling evidence from murine models of cancer and early clinical trials in support of macrophage-targeted intervention strategies with the potential to dramatically reduce breast cancer morbidity and mortality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8, 98 (1989). - PubMed
-
- Kroemer, G. et al. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 21, 1128–1138 (2015). - PubMed
-
- Koebel, C. M. et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907 (2007). - PubMed
-
- Denkert, C. et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 28, 105–113 (2010). - PubMed
-
- Ali, H. R. et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 25, 1536–1543 (2014). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

