The Soluble Guanylate Cyclase Stimulator IWP-953 Increases Conventional Outflow Facility in Mouse Eyes
- PMID: 26998718
- PMCID: PMC4811179
- DOI: 10.1167/iovs.15-18958
The Soluble Guanylate Cyclase Stimulator IWP-953 Increases Conventional Outflow Facility in Mouse Eyes
Abstract
Purpose: The nitric oxide (NO)-cyclic guanosine-3',5'-monophosphate (cGMP) pathway regulates aqueous humor outflow and therefore, intraocular pressure. We investigated the pharmacologic effects of the soluble guanylate cyclase (sGC) stimulator IWP-953 on primary human trabecular meshwork (HTM) cells and conventional outflow facility in mouse eyes.
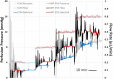
Methods: Cyclic GMP levels were determined in vitro in HEK-293 cells and four HTM cell strains (HTM120/HTM123: predominantly myofibroblast-like phenotype, HTM130/HTM141: predominantly endothelial-like phenotype), and in HTM cell culture supernatants. Conventional outflow facility was measured following intracameral injection of IWP-953 or DETA-NO using a computerized pressure-controlled perfusion system in enucleated mouse eyes ex vivo.
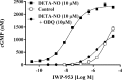
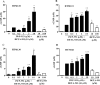
Results: IWP-953 markedly stimulated cGMP production in HEK-293 cells in the presence and absence of DETA-NO (half maximal effective concentrations: 17 nM, 9.5 μM). Similarly, IWP-953 stimulated cGMP production in myofibroblast-like HTM120 and HTM123 cells, an effect that was greatly amplified by the presence of DETA-NO. In contrast, IWP-953 stimulation of cGMP production in endothelial-like HTM130 and HTM141 cells was observed, but was markedly less prominent than in HTM120 and HTM123 cells. Notably, cGMP was found in all HTM culture supernatants, following IWP-953/DETA-NO stimulation. In paired enucleated mouse eyes, IWP-953 at 10, 30, 60, and 100 μM concentration-dependently increased outflow facility. This effect (89.5%) was maximal at 100 μM (P = 0.002) and in magnitude comparable to DETA-NO at 100 μM (97.5% increase, P = 0.030).
Conclusions: These data indicate that IWP-953, via modulation of the sGC-cGMP pathway, increases aqueous outflow facility in mouse eyes, suggesting therapeutic potential for sGC stimulators as novel ocular hypotensive drugs.
Figures


References
-
- Kass MA,, Heuer DK,, Higginbotham EJ,, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701–713. - PubMed
-
- Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963; 69: 783–801. - PubMed
-
- Challa P,, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Invest Drugs. 2014; 23: 81–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

