The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of miR-7a on Its Target Genes Expression
- PMID: 26998750
- PMCID: PMC4801407
- DOI: 10.1371/journal.pone.0151753
The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of miR-7a on Its Target Genes Expression
Abstract
Objectives: Recent studies have demonstrated the role of Cdr1as (or CiRS-7), one of the well-identified circular RNAs (circRNAs), as a miR-7a/b sponge or inhibitor in brain tissues or islet cells. This study aimed to investigate the presence of Cdr1as/miR-7a pathway in cardiomyocytes, and explore the mechanism underlying the function of miR-7a in protecting against myocardial infarction (MI)-induced apoptosis.
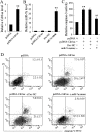
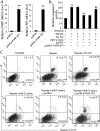
Methods: Mouse MI injury model was established and evaluated by infarct size determination. Real-time PCR was performed to quantify the expression of Cdr1as and miR-7a in cardiomyocytes. Cell apoptosis was determined by caspase-3 activity analysis and flow cytometry assays with Annexin V/PI staining. Transfection of Cdr1as overexpressing plasmid and miR-7a mimic were conducted for gain-of-function studies. Luciferase reporter assay and western blot analysis were performed to verity potential miR-7a targets.
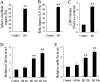
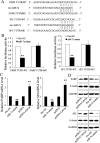
Results: Cdr1as and miR-7a were both upregulated in MI mice with increased cardiac infarct size, or cardiomyocytes under hypoxia treatment. Cdr1as overexpression in MCM cells promoted cell apoptosis, but was then reversed by miR-7a overexpression. The SP1 was identified as a new miR-7a target, in line with previously identified PARP, while miR-7a-induced decrease of cell apoptosis under hypoxia treatment was proven to be inhibited by PARP-SP1 overexpression. Moreover, Cdr1as overexpression in vivo increased cardiac infarct size with upregulated expression of PARP and SP1, while miR-7a overexpression reversed these changes.
Conclusions: Cdr1as also functioned as a powerful miR-7a sponge in myocardial cells, and showed regulation on the protective role of miR-7a in MI injury, involving the function of miR-7a targets, PARP and SP1.
Conflict of interest statement
Figures

References
-
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiological reviews. 1999;79(1):215–62. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

