Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques
- PMID: 26998834
- PMCID: PMC4983100
- DOI: 10.1038/nm.4063
Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques
Abstract
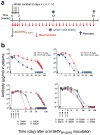
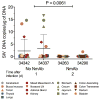
Prevention of mother-to-child transmission (MTCT) of HIV remains a major objective where antenatal care is not readily accessible. We tested HIV-1-specific human neutralizing monoclonal antibodies (NmAbs) as a post-exposure therapy in an infant macaque model for intrapartum MTCT. One-month-old rhesus macaques were inoculated orally with the simian-human immunodeficiency virus SHIVSF162P3. On days 1, 4, 7 and 10 after virus exposure, we injected animals subcutaneously with NmAbs and quantified systemic distribution of NmAbs in multiple tissues within 24 h after antibody administration. Replicating virus was found in multiple tissues by day 1 in animals that were not treated. All NmAb-treated macaques were free of virus in blood and tissues at 6 months after exposure. We detected no anti-SHIV T cell responses in blood or tissues at necropsy, and no virus emerged after CD8(+) T cell depletion. These results suggest that early passive immunotherapy can eliminate early viral foci and thereby prevent the establishment of viral reservoirs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Mofenson LM. Prevention of mother-to-child HIV-1 transmission--why we still need a preventive HIV immunization strategy. J Acquir Immune Defic Syndr. 2011;58:359–362. - PubMed
-
- Safrit JT, et al. Immunoprophylaxis to prevent mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2004;35:169–177. - PubMed
-
- Kumwenda NI, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

