The Structure and Function of Type III Secretion Systems
- PMID: 26999392
- PMCID: PMC4804468
- DOI: 10.1128/microbiolspec.VMBF-0004-2015
The Structure and Function of Type III Secretion Systems
Abstract
Type III secretion systems (T3SSs) afford Gram-negative bacteria an intimate means of altering the biology of their eukaryotic hosts--the direct delivery of effector proteins from the bacterial cytoplasm to that of the eukaryote. This incredible biophysical feat is accomplished by nanosyringe "injectisomes," which form a conduit across the three plasma membranes, peptidoglycan layer, and extracellular space that form a barrier to the direct delivery of proteins from bacterium to host. The focus of this chapter is T3SS function at the structural level; we will summarize the core findings that have shaped our understanding of the structure and function of these systems and highlight recent developments in the field. In turn, we describe the T3SS secretory apparatus, consider its engagement with secretion substrates, and discuss the posttranslational regulation of secretory function. Lastly, we close with a discussion of the future prospects for the interrogation of structure-function relationships in the T3SS.
Figures
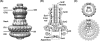
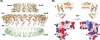

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

