CsoR Is Essential for Maintaining Copper Homeostasis in Mycobacterium tuberculosis
- PMID: 26999439
- PMCID: PMC4801387
- DOI: 10.1371/journal.pone.0151816
CsoR Is Essential for Maintaining Copper Homeostasis in Mycobacterium tuberculosis
Abstract
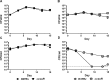
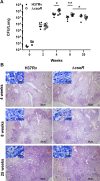
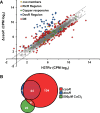
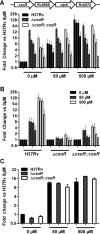
Mycobacterium tuberculosis, a pathogen infecting one third of the world population, faces numerous challenges within the host, including high levels of copper. We have previously shown that M. tuberculosis CsoR is a copper inducible transcriptional regulator. Here we examined the hypothesis that csoR is necessary for maintaining copper homeostasis and surviving under various stress conditions. With an unmarked csoR knockout strain, we were able to characterize the role of csoR in M. tuberculosis as it faced copper and host stress. Growth under high levels of copper demonstrated that M. tuberculosis survives copper stress significantly better in the absence of csoR. Yet under minimal levels of copper, differential expression analysis revealed that the loss of csoR results in a cell wide hypoxia-type stress response with the induction of the DosR regulon. Despite the stress placed on M. tuberculosis by the loss of csoR, survival of the knockout strain was increased compared to wild type during the early chronic stages of mouse infection, suggesting that csoR could play an active role in modulating M. tuberculosis fitness within the host. Overall, analysis of CsoR provided an increased understanding of the M. tuberculosis copper response with implications for other intracellular pathogens harboring CsoR.
Conflict of interest statement
Figures


References
-
- World Health Organization (WHO). Global tuberculosis report 2013 Geneva: WHO; 2013. October 23.
-
- Adams LB, Dinauer MC, Morgenstern DE, Krahenbuhl JL. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber Lung Dis 1997; 78: 237–246. - PubMed
-
- Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol 1998; 160: 1290–1296. - PubMed
-
- Wagner D, Maser J, Lai B, Cai Z, Barry CE III, Honer Zu BK, et al. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J Immunol 2005; 174: 1491–1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

