Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor
- PMID: 26999600
- PMCID: PMC4811124
- DOI: 10.1172/JCI82771
Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor
Abstract
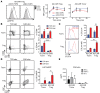
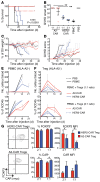
Adoptive immunotherapy with regulatory T cells (Tregs) is a promising treatment for allograft rejection and graft-versus-host disease (GVHD). Emerging data indicate that, compared with polyclonal Tregs, disease-relevant antigen-specific Tregs may have numerous advantages, such as a need for fewer cells and reduced risk of nonspecific immune suppression. Current methods to generate alloantigen-specific Tregs rely on expansion with allogeneic antigen-presenting cells, which requires access to donor and recipient cells and multiple MHC mismatches. The successful use of chimeric antigen receptors (CARs) for the generation of antigen-specific effector T cells suggests that a similar approach could be used to generate alloantigen-specific Tregs. Here, we have described the creation of an HLA-A2-specific CAR (A2-CAR) and its application in the generation of alloantigen-specific human Tregs. In vitro, A2-CAR-expressing Tregs maintained their expected phenotype and suppressive function before, during, and after A2-CAR-mediated stimulation. In mouse models, human A2-CAR-expressing Tregs were superior to Tregs expressing an irrelevant CAR at preventing xenogeneic GVHD caused by HLA-A2+ T cells. Together, our results demonstrate that use of CAR technology to generate potent, functional, and stable alloantigen-specific human Tregs markedly enhances their therapeutic potential in transplantation and sets the stage for using this approach for making antigen-specific Tregs for therapy of multiple diseases.
Figures






Comment in
-
Driving allotolerance: CAR-expressing Tregs for tolerance induction in organ and stem cell transplantation.J Clin Invest. 2016 Apr 1;126(4):1248-50. doi: 10.1172/JCI86827. Epub 2016 Mar 21. J Clin Invest. 2016. PMID: 26999608 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

