Preterm Birth and Low Birth Weight after In Utero Exposure to Antiretrovirals Initiated during Pregnancy in Yaoundé, Cameroon
- PMID: 26999744
- PMCID: PMC4801361
- DOI: 10.1371/journal.pone.0150565
Preterm Birth and Low Birth Weight after In Utero Exposure to Antiretrovirals Initiated during Pregnancy in Yaoundé, Cameroon
Abstract
Background: Effects of antiretroviral therapy (ART) on birth outcomes remain controversial.
Objective: To assess the impact of antenatal exposure to ART on the occurrence of preterm birth (PTB) and low birth weight (LBW).
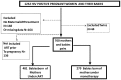
Methods: A cross-sectional study conducted at the Essos Hospital Center in Yaounde from 2008 to 2011 among HIV vertically exposed infants with two distinct maternal antiretroviral experiences: monotherapy group (Zidovudine, ZDV) and the combination ART group (cART). Mothers already receiving cART before pregnancy were ineligible. In both groups, events of PTB (<37 weeks) and LBW (<2,500g) were analyzed using univariate and multivariate logistic regression; with p<0.05 considered statistically significant.
Results: Of the 760 infants, 481 were born from cART-exposed mothers against 279 from maternal-ZDV. Median maternal CD4 count was 378 [interquartile range (IQR): 253-535] cells/mm3. Median duration of ART at onset of delivery was 13 [IQR: 10-17] weeks. In the cART-group, 64.9% (312/481) of mothers were exposed to Zidovudine/Lamuvidine/Nevirapine and only 2% (9/481) were on protease inhibitor-based regimens. Events of PTB were not significantly higher in the cART-group compared to the ZDV-group (10.2% vs. 6.4% respectively, p = 0.08), while onsets of LBW were significantly found in the cART-group compared to ZDV-group (11.6% vs. 7.2% respectively, p = 0.05). Other factors (parity, maternal age at delivery or CD4 cell count) were not associated with PTB.
Conclusion: cART, initiated during pregnancy, would be an independent factor of LBW. In the era of option B+ (lifelong ART to all HIV-pregnant women), further studies would guide towards measures limiting onsets of LBW.
Conflict of interest statement
Figures
References
-
- de Vincenzi I (2011) Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 3: 171–80 - PubMed
-
- UNAIDS (2011) Global plan towards the elimination of new HIVinfections among children by 2015 and keeping their mothers alive Geneva: UNAIDS. - PubMed
-
- World Health Organization (2010) Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Recommandations for a public health approach. Geneva. - PubMed
-
- Ministry of Public Health (2009) Recommandations for use of antiretroviral drugs during pregnancy Ministry of Public Health, Yaounde.
-
- Njom Nlend A E, Ekobo C Same, Bitoungui M J, Ekani B Bagfegue, Tchokoteu P, Lyeb S, et al. (2012) Early outcomes of HIV exposed children in the first district-wide programme using extended regimens for the prevention of mother-to-child transmission of HIV, in Yaounde, Cameroon. J Trop Pediatr 4: 297–302 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials