Valosin-containing protein is a key mediator between autophagic cell death and apoptosis in adult hippocampal neural stem cells following insulin withdrawal
- PMID: 27000202
- PMCID: PMC4802725
- DOI: 10.1186/s13041-016-0212-8
Valosin-containing protein is a key mediator between autophagic cell death and apoptosis in adult hippocampal neural stem cells following insulin withdrawal
Abstract
Background: Programmed cell death (PCD) plays essential roles in the regulation of survival and function of neural stem cells (NSCs). Abnormal regulation of this process is associated with developmental and degenerative neuronal disorders. However, the mechanisms underlying the PCD of NSCs remain largely unknown. Understanding the mechanisms of PCD in NSCs is crucial for exploring therapeutic strategies for the treatment of neurodegenerative diseases.
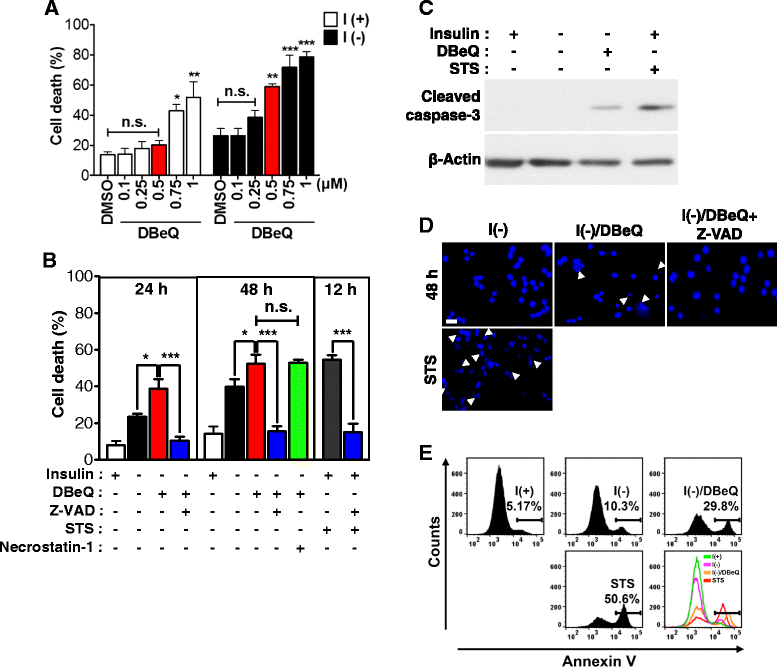
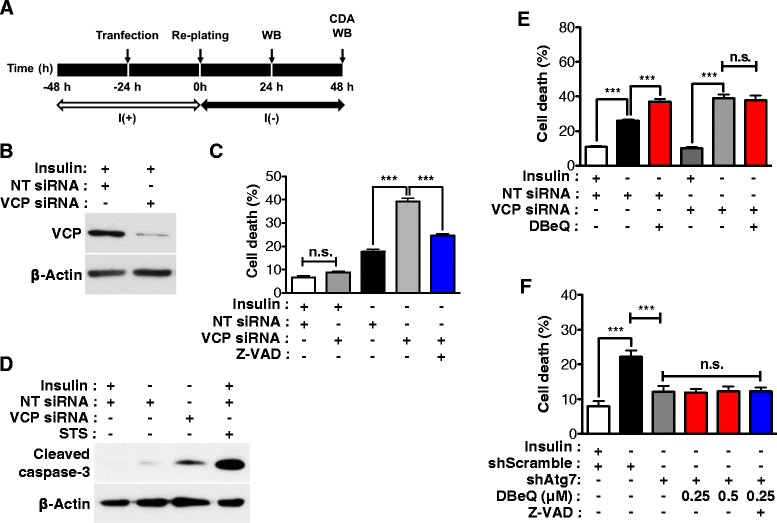
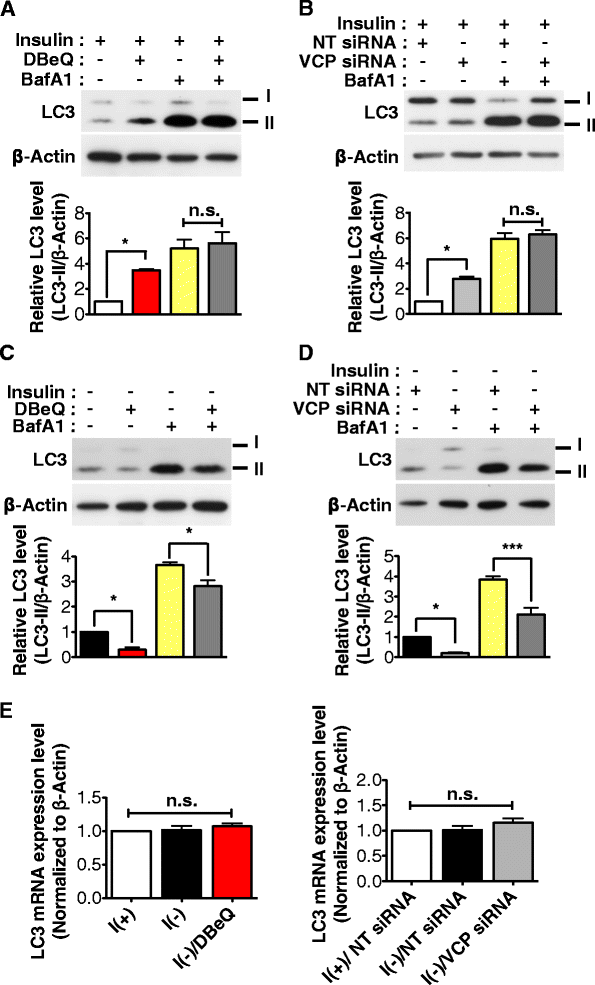
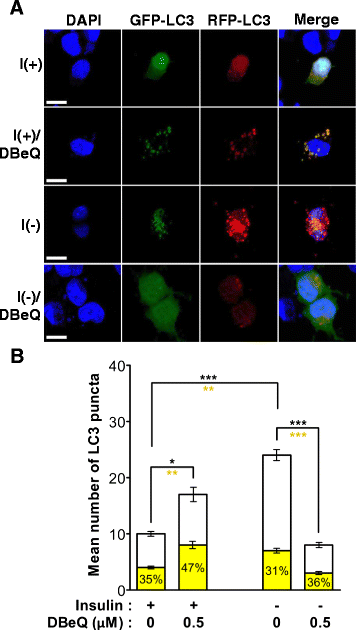
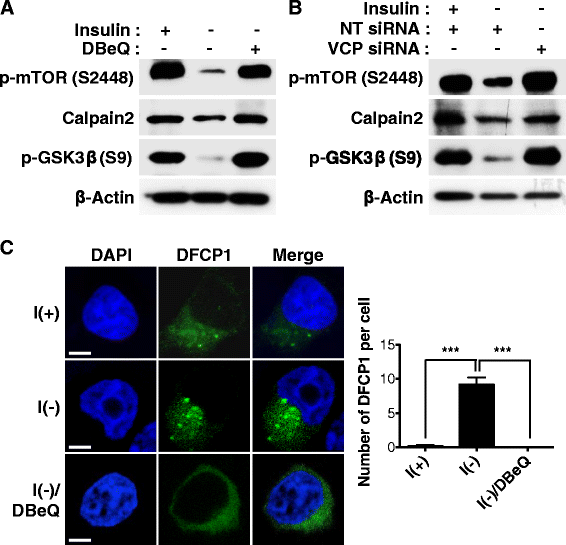
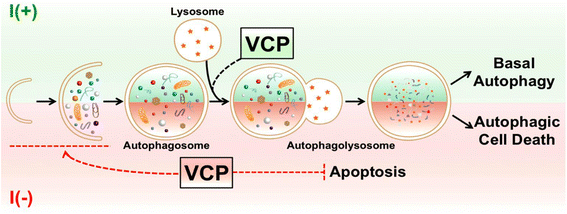
Result: We have previously reported that adult rat hippocampal neural stem (HCN) cells undergo autophagic cell death (ACD) following insulin withdrawal without apoptotic signs despite their normal apoptotic capabilities. It is unknown how interconnection between ACD and apoptosis is mediated in HCN cells. Valosin-containing protein (VCP) is known to be essential for autophagosome maturation in mammalian cells. VCP is abundantly expressed in HCN cells compared to hippocampal tissue and neurons. Pharmacological and genetic inhibition of VCP at basal state in the presence of insulin modestly impaired autophagic flux, consistent with its known role in autophagosome maturation. Of note, VCP inaction in insulin-deprived HCN cells significantly decreased ACD and down-regulated autophagy initiation signals with robust induction of apoptosis. Overall autophagy level was also substantially reduced, suggesting the novel roles of VCP at initial step of autophagy.
Conclusion: Taken together, these data demonstrate that VCP may play an essential role in the initiation of autophagy and mediation of crosstalk between ACD and apoptosis in HCN cells when autophagy level is high upon insulin withdrawal. This is the first report on the role of VCP in regulation of NSC cell death. Elucidating the mechanism by which VCP regulates the crosstalk of ACD and apoptosis will contribute to understanding the molecular mechanism of PCD in NSCs.
Keywords: Adult neural stem cells; Apoptosis; Autophagic cell death; Insulin withdrawal; Valosin-containing protein.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

