Abiraterone acetate in metastatic castration-resistant prostate cancer - the unanticipated real-world clinical experience
- PMID: 27001043
- PMCID: PMC4802641
- DOI: 10.1186/s12894-016-0132-z
Abiraterone acetate in metastatic castration-resistant prostate cancer - the unanticipated real-world clinical experience
Abstract
Background: There is much interest in confirming whether the efficacy of abiraterone acetate (AA) demonstrated within the trial setting is reproducible in routine clinical practice. We report the clinical outcome of metastatic castration-resistant prostate cancer (mCRPC) patients treated with AA in real-life clinical practice.
Methods: The clinical records of mCRPC patients treated with AA from all 6 public oncology centers in Hong Kong between August 2011 and December 2014 were reviewed. The treatment efficacy and its determinants, and toxicities were determined.
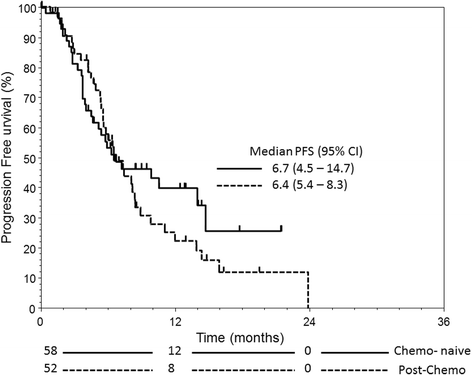
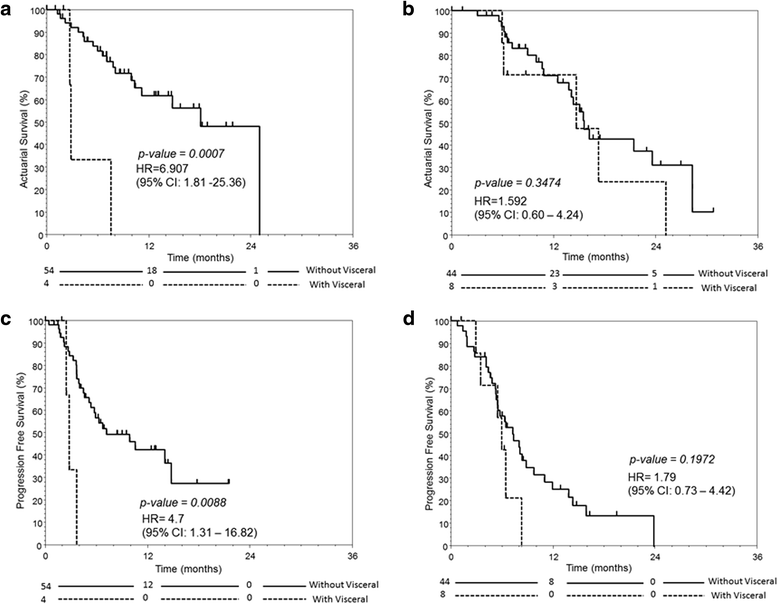
Results: A total of 110 patients with mCRPC were treated with AA in the review period, of whom 58 were chemo-naive and 52 had received prior chemotherapy (post-chemo). The median follow-up time was 7.5/11.4 months for chemo-naive/post-chemo patients. 6.9/15.4 % of chemo-naive/post-chemo patients had visceral metastases. The median overall survival (OS) and progression-free survival (PFS) were 18.1/15.5 months and 6.7/6.4 months for chemo-naive/post-chemo patients, respectively. Among chemo-naive patients, those with visceral diseases had significantly inferior OS (2.8 vs 18.0 p = 0.0007) and PFS (2.8 vs 6.8 months, p = 0.0088) than those without. Pain control was comparable in both groups of patients. The most common grade 3 or above toxicities were hypertension (6.9/5.8 %) and hypokalemia (3.4/3.8 %) in chemo-naive/post-chemo patients. In multivariate analysis, the presence of prostate-specific antigen (PSA) response (≥50 % drop of PSA from baseline) within the first 3 months of therapy was associated with favorable OS and PFS in both chemo-naive and post-chemo group.
Conclusions: In clinical practice outside the trial setting, OS after AA in our chemo-naive patient cohort (18.1 months) was considerably shorter than that reported in the COU-AA-302 trial (34.7 months), and the OS was particularly short in those with visceral metastases (2.8 months). Conversely, AA was efficacious in post-chemo patients. AA resulted in comparable pain control in both groups of patients. The presence of PSA response within the first 3 months of treatment was a significant determinant of survival.
Keywords: Abiraterone acetate; Castration-resistant prostate cancer; Chemo-naïve; Chemotherapy; PSA response.
Figures

References
-
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. - DOI - PubMed
-
- Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN, Garrels K, Hotte S, Kattan MW, Raghavan D, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32(30):3436–48. doi: 10.1200/JCO.2013.54.8404. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

