Chondroinduction from Naturally Derived Cartilage Matrix: A Comparison Between Devitalized and Decellularized Cartilage Encapsulated in Hydrogel Pastes
- PMID: 27001140
- PMCID: PMC4840832
- DOI: 10.1089/ten.TEA.2015.0546
Chondroinduction from Naturally Derived Cartilage Matrix: A Comparison Between Devitalized and Decellularized Cartilage Encapsulated in Hydrogel Pastes
Abstract
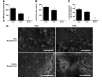
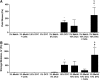
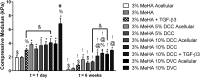
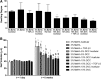
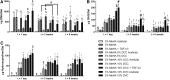
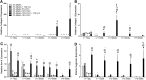
Hydrogel precursors are liquid solutions that are prone to leaking after surgical placement. This problem was overcome by incorporating either decellularized cartilage (DCC) or devitalized cartilage (DVC) microparticles into traditional photocrosslinkable hydrogel precursors in an effort to achieve a paste-like hydrogel precursor. DCC and DVC were selected specifically for their potential to induce chondrogenesis of stem cells, given that materials that are chondroinductive on their own without growth factors are a revolutionary goal in orthopedic medicine. We hypothesized that DVC, lacking the additional chemical processing steps in DCC to remove cell content, would lead to a more chondroinductive hydrogel with rat bone marrow-derived mesenchymal stem cells. Hydrogels composed of methacrylated hyaluronic acid (MeHA) and either DCC or DVC microparticles were tested with and without exposure to transforming growth factor (TGF)-β3 over a 6 week culture period, where swelling, mechanical analysis, and gene expression were observed. For collagen II, Sox-9, and aggrecan expression, MeHA precursors containing DVC consistently outperformed the DCC-containing groups, even when the DCC groups were exposed to TGF-β3. DVC consistently outperformed all TGF-β3-exposed groups in aggrecan and collagen II gene expression as well. In addition, when the same concentrations of MeHA with DCC or DVC microparticles were evaluated for yield stress, the yield stress with the DVC microparticles was 2.7 times greater. Furthermore, the only MeHA-containing group that exhibited shape retention was the group containing DVC microparticles. DVC appeared to be superior to DCC in both chondroinductivity and rheological performance of hydrogel precursors, and therefore DVC microparticles may hold translational potential for cartilage regeneration.
Figures


References
-
- Rughani R.V., Branco M.C., Pochan D.J., and Schneider J.P. De novo design of a shear-thin recoverable peptide-based hydrogel capable of intrafibrillar photopolymerization. Macromolecules 43, 7924, 2010
-
- Todd R.H., and Daniel S.K. Hydrogels in drug delivery: progress and challenges. Polymer 49, 1993, 2008
-
- Benders K., van Weeren P., Badylak S., Saris D., Dhert W., and Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 31, 169, 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

