Improved dialytic removal of protein-bound uraemic toxins with use of albumin binding competitors: an in vitro human whole blood study
- PMID: 27001248
- PMCID: PMC4802219
- DOI: 10.1038/srep23389
Improved dialytic removal of protein-bound uraemic toxins with use of albumin binding competitors: an in vitro human whole blood study
Abstract
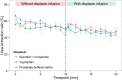
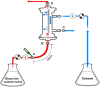
Protein-bound uraemic toxins (PBUTs) cause various deleterious effects in end-stage kidney disease patients, because their removal by conventional haemodialysis (HD) is severely limited by their low free fraction in plasma. Here we provide an experimental validation of the concept that the HD dialytic removal of PBUTs can be significantly increased by extracorporeal infusion of PBUT binding competitors. The binding properties of indoxyl sulfate (IS), indole-3-acetic acid (IAA) and hippuric acid (HIPA) and their binding competitors, ibuprofen (IBU), furosemide (FUR) and tryptophan (TRP) were studied in uraemic plasma. The effect of binding competitor infusion on fractional removal of PBUT was then quantified in an ex vivo single-pass HD model using uraemic human whole blood. The infusion of a combination of IBU and FUR increased the fractional removal of IS from 6.4 ± 0.1 to 18.3 ± 0.4%. IAA removal rose from 16.8 ± 0.3 to 34.5 ± 0.7%. TRP infusion increased the removal of IS and IAA to 10.5 ± 0.1% and 27.1 ± 0.3%, respectively. Moderate effects were observed on HIPA removal. Pre-dialyzer infusion of PBUT binding competitors into the blood stream can increase the HD removal of PBUTs. This approach can potentially be applied in current HD settings.
Conflict of interest statement
Xia Tao, Stephan Thijssen and Peter Kotanko are employees of Renal Research Institute, affiliated with Fresenius Medical Care North America (FMCNA). Peter Kotanko holds stock of FMCNA. Peter Kotanko is an inventor on a U.S. patent (assigned to FMCNA) on the method described in the paper. Chih-Hu Ho, Michael Henrie and Eric Stroup are employees of FMCNA. Garry Handelman declares no conflicts of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

