Involvement of budding yeast Rad5 in translesion DNA synthesis through physical interaction with Rev1
- PMID: 27001510
- PMCID: PMC4914093
- DOI: 10.1093/nar/gkw183
Involvement of budding yeast Rad5 in translesion DNA synthesis through physical interaction with Rev1
Abstract
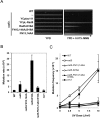
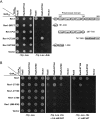
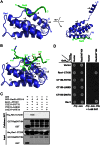
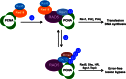
DNA damage tolerance (DDT) is responsible for genomic stability and cell viability by bypassing the replication block. In Saccharomyces cerevisiae DDT employs two parallel branch pathways to bypass the DNA lesion, namely translesion DNA synthesis (TLS) and error-free lesion bypass, which are mediated by sequential modifications of PCNA. Rad5 has been placed in the error-free branch of DDT because it contains an E3 ligase domain required for PCNA polyubiquitination. Rad5 is a multi-functional protein and may also play a role in TLS, since it interacts with the TLS polymerase Rev1. In this study we mapped the Rev1-interaction domain in Rad5 to the amino acid resolution and demonstrated that Rad5 is indeed involved in TLS possibly through recruitment of Rev1. Genetic analyses show that the dual functions of Rad5 can be separated and reconstituted. Crystal structure analysis of the Rad5-Rev1 interaction reveals a consensus RFF motif in the Rad5 N-terminus that binds to a hydrophobic pocket within the C-terminal domain of Rev1 that is highly conserved in eukaryotes. This study indicates that Rad5 plays a critical role in pathway choice between TLS and error-free DDT.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Prakash L. Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol. Gen. Genet. 1981;184:471–478. - PubMed
-
- Broomfield S., Hryciw T., Xiao W. DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res. 2001;486:167–184. - PubMed
-
- Lawrence C.W. Cellular functions of DNA polymerase zeta and Rev1 protein. Adv. Protein Chem. 2004;69:167–203. - PubMed
-
- Prakash L. The RAD6 gene and protein of Saccharomyces cerevisiae. Ann. N.Y. Acad. Sci. 1994;726:267–273. - PubMed
-
- Hoege C., Pfander B., Moldovan G.L., Pyrowolakis G., Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

