Staphylococcus aureus Phenol-Soluble Modulins Impair Interleukin Expression in Bovine Mammary Epithelial Cells
- PMID: 27001539
- PMCID: PMC4907149
- DOI: 10.1128/IAI.01330-15
Staphylococcus aureus Phenol-Soluble Modulins Impair Interleukin Expression in Bovine Mammary Epithelial Cells
Abstract
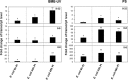
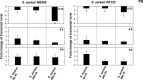
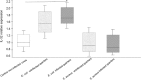
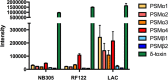
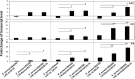
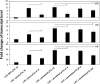
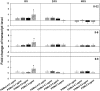
The role of the recently described interleukin-32 (IL-32) in Staphylococcus aureus-induced mastitis, an inflammation of the mammary gland, is unclear. We determined expression of IL-32, IL-6, and IL-8 in S. aureus- and Escherichia coli-infected bovine mammary gland epithelial cells. Using live bacteria, we found that in S. aureus-infected cells, induction of IL-6 and IL-8 expression was less pronounced than in E. coli-infected cells. Notably, IL-32 expression was decreased in S. aureus-infected cells, while it was increased in E. coli-infected cells. We identified the staphylococcal phenol-soluble modulin (PSM) peptides as key contributors to these effects, as IL-32, IL-6, and IL-8 expression by epithelial cells exposed to psm mutant strains was significantly increased compared to that in cells exposed to the isogenic S. aureus wild-type strain, indicating that PSMs inhibit the production of these interleukins. The use of genetically complemented strains confirmed this observation. Inasmuch as the decreased expression of IL-32, which is involved in dendritic cell maturation, impairs immune responses, our results support a PSM-dependent mechanism that allows for the development of chronic S. aureus-related mastitis.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-kappaB in mammary epithelial cells and to quickly induce TNFalpha and interleukin-8 (CXCL8) expression in the udder.Mol Immunol. 2008 Mar;45(5):1385-97. doi: 10.1016/j.molimm.2007.09.004. Epub 2007 Oct 22. Mol Immunol. 2008. PMID: 17936907
-
Differentiating Staphylococcus aureus from Escherichia coli mastitis: S. aureus triggers unbalanced immune-dampening and host cell invasion immediately after udder infection.Sci Rep. 2017 Jul 6;7(1):4811. doi: 10.1038/s41598-017-05107-4. Sci Rep. 2017. PMID: 28684793 Free PMC article.
-
Differential cytokine and chemokine responses of bovine mammary epithelial cells to Staphylococcus aureus and Escherichia coli.Cytokine. 2007 Apr;38(1):12-21. doi: 10.1016/j.cyto.2007.04.006. Epub 2007 May 25. Cytokine. 2007. PMID: 17532224
-
Phenol-soluble modulins--critical determinants of staphylococcal virulence.FEMS Microbiol Rev. 2014 Jul;38(4):698-719. doi: 10.1111/1574-6976.12057. Epub 2014 Jan 16. FEMS Microbiol Rev. 2014. PMID: 24372362 Free PMC article. Review.
-
Genetic studies of Staphylococcus aureus virulence factors.Antonie Van Leeuwenhoek. 1988;54(5):475-82. doi: 10.1007/BF00461866. Antonie Van Leeuwenhoek. 1988. PMID: 3060011 Review. No abstract available.
Cited by
-
Progress towards the Elusive Mastitis Vaccines.Vaccines (Basel). 2022 Feb 15;10(2):296. doi: 10.3390/vaccines10020296. Vaccines (Basel). 2022. PMID: 35214754 Free PMC article. Review.
-
Virulence Mechanisms of Staphylococcal Animal Pathogens.Int J Mol Sci. 2023 Sep 26;24(19):14587. doi: 10.3390/ijms241914587. Int J Mol Sci. 2023. PMID: 37834035 Free PMC article. Review.
-
Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: current understanding and future perspectives.BMC Vet Res. 2022 Mar 24;18(1):115. doi: 10.1186/s12917-022-03197-5. BMC Vet Res. 2022. PMID: 35331225 Free PMC article. Review.
-
Cellular and soluble components decrease the viable pathogen counts in milk from dairy cows with subclinical mastitis.J Vet Med Sci. 2017 Aug 10;79(8):1389-1393. doi: 10.1292/jvms.17-0269. Epub 2017 Jul 7. J Vet Med Sci. 2017. PMID: 28690278 Free PMC article.
-
Preconditioning with Lipopolysaccharide or Lipoteichoic Acid Protects against Staphylococcus aureus Mammary Infection in Mice.Front Immunol. 2017 Jul 24;8:833. doi: 10.3389/fimmu.2017.00833. eCollection 2017. Front Immunol. 2017. PMID: 28791009 Free PMC article.
References
-
- Schukken YH, Gunther J, Fitzpatrick J, Fontaine MC, Goetze L, Holst O, Leigh J, Petzl W, Schuberth HJ, Sipka A, Smith DG, Quesnell R, Watts J, Yancey R, Zerbe H, Gurjar A, Zadoks RN, Seyfert HM. 2011. Host-response patterns of intramammary infections in dairy cows. Vet Immunol Immunopathol 144:270–289. doi:10.1016/j.vetimm.2011.08.022. - DOI - PubMed
-
- Fu Y, Zhou E, Liu Z, Li F, Liang D, Liu B, Song X, Zhao F, Fen X, Li D, Cao Y, Zhang X, Zhang N, Yang Z. 2013. Staphylococcus aureus and Escherichia coli elicit different innate immune responses from bovine mammary epithelial cells. Vet Immunol Immunopathol 155:245–252. doi:10.1016/j.vetimm.2013.08.003. - DOI - PubMed
-
- Jensen K, Gunther J, Talbot R, Petzl W, Zerbe H, Schuberth HJ, Seyfert HM, Glass EJ. 2013. Escherichia coli- and Staphylococcus aureus-induced mastitis differentially modulate transcriptional responses in neighbouring uninfected bovine mammary gland quarters. BMC Genomics 14:36. doi:10.1186/1471-2164-14-36. - DOI - PMC - PubMed
-
- Alekseeva L, Huet D, Femenia F, Mouyna I, Abdelouahab M, Cagna A, Guerrier D, Tichanne-Seltzer V, Baeza-Squiban A, Chermette R, Latge JP, Berkova N. 2009. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol 9:33. doi:10.1186/1471-2180-9-33. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

