Ketamine-Induced Apoptosis in Normal Human Urothelial Cells: A Direct, N-Methyl-d-Aspartate Receptor-Independent Pathway Characterized by Mitochondrial Stress
- PMID: 27001627
- PMCID: PMC4861758
- DOI: 10.1016/j.ajpath.2015.12.014
Ketamine-Induced Apoptosis in Normal Human Urothelial Cells: A Direct, N-Methyl-d-Aspartate Receptor-Independent Pathway Characterized by Mitochondrial Stress
Abstract
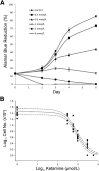
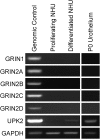
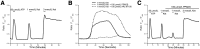
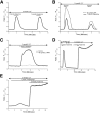
Recreational abuse of ketamine has been associated with the emergence of a new bladder pain syndrome, ketamine-induced cystitis, characterized by chronic inflammation and urothelial ulceration. We investigated the direct effects of ketamine on normal human urothelium maintained in organ culture or as finite cell lines in vitro. Exposure of urothelium to ketamine resulted in apoptosis, with cytochrome c release from mitochondria and significant subsequent caspase 9 and 3/7 activation. The anesthetic mode-of-action for ketamine is mediated primarily through N-methyl d-aspartate receptor (NMDAR) antagonism; however, normal (nonimmortalized) human urothelial cells were unresponsive to NMDAR agonists or antagonists, and no expression of NMDAR transcript was detected. Exposure to noncytotoxic concentrations of ketamine (≤1 mmol/L) induced rapid release of ATP, which activated purinergic P2Y receptors and stimulated the inositol trisphosphate receptor to provoke transient release of calcium from the endoplasmic reticulum into the cytosol. Ketamine concentrations >1 mmol/L were cytotoxic and provoked a larger-amplitude increase in cytosolic Ca(2+) concentration that was unresolved. The sustained elevation in cytosolic Ca(2+) concentration was associated with pathological mitochondrial oxygen consumption and ATP deficiency. Damage to the urinary barrier initiates bladder pain and, in ketamine-induced cystitis, loss of urothelium from large areas of the bladder wall is a reported feature. This study offers first evidence for a mechanism of direct toxicity of ketamine to urothelial cells by activating the intrinsic apoptotic pathway.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Wood D., Cottrell A., Baker S.C., Southgate J., Harris M., Fulford S., Woodhouse C., Gillatt D. Recreational ketamine: from pleasure to pain. BJU Int. 2011;107:1881–1884. - PubMed
-
- Shahani R., Streutker C., Dickson B., Stewart R.J. Ketamine-associated ulcerative cystitis: a new clinical entity. Urology. 2007;69:810–812. - PubMed
-
- Chu P.S., Kwok S.C., Lam K.M., Chu T.Y., Chan S.W., Man C.W., Ma W.K., Chui K.L., Yiu M.K., Chan Y.C., Tse M.L., Lau F.L. “Street ketamine”-associated bladder dysfunction: a report of ten cases. Hong Kong Med J. 2007;13:311–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

