The macrophage IRF8/IRF1 regulome is required for protection against infections and is associated with chronic inflammation
- PMID: 27001747
- PMCID: PMC4821649
- DOI: 10.1084/jem.20151764
The macrophage IRF8/IRF1 regulome is required for protection against infections and is associated with chronic inflammation
Abstract
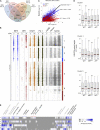
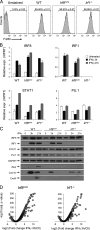
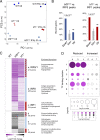
IRF8 and IRF1 are transcriptional regulators that play critical roles in the development and function of myeloid cells, including activation of macrophages by proinflammatory signals such as interferon-γ (IFN-γ). Loss of IRF8 or IRF1 function causes severe susceptibility to infections in mice and in humans. We used chromatin immunoprecipitation sequencing and RNA sequencing in wild type and inIRF8andIRF1mutant primary macrophages to systematically catalog all of the genes bound by (cistromes) and transcriptionally activated by (regulomes) IRF8, IRF1, PU.1, and STAT1, including modulation of epigenetic histone marks. Of the seven binding combinations identified, two (cluster 1 [IRF8/IRF1/STAT1/PU.1] and cluster 5 [IRF1/STAT1/PU.1]) were found to have a major role in controlling macrophage transcriptional programs both at the basal level and after IFN-γ activation. They direct the expression of a set of genes, the IRF8/IRF1 regulome, that play critical roles in host inflammatory and antimicrobial defenses in mouse models of neuroinflammation and of pulmonary tuberculosis, respectively. In addition, this IRF8/IRF1 regulome is enriched for genes mutated in human primary immunodeficiencies and with loci associated with several inflammatory diseases in humans.
© 2016 Langlais et al.
Figures






References
-
- Abdollahi A., Lord K.A., Hoffman-Liebermann B., and Liebermann D.A.. 1991. Interferon regulatory factor 1 is a myeloid differentiation primary response gene induced by interleukin 6 and leukemia inhibitory factor: role in growth inhibition. Cell Growth Differ. 2:401–407. - PubMed
-
- Al-Herz W., Bousfiha A., Casanova J.-L., Chatila T., Conley M.E., Cunningham-Rundles C., Etzioni A., Franco J.L., Gaspar H.B., Holland S.M., et al. 2014. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front. Immunol. 5:162. - PMC - PubMed
-
- Beecham A.H., Patsopoulos N.A., Xifara D.K., Davis M.F., Kemppinen A., Cotsapas C., Shah T.S., Spencer C., Booth D., Goris A., et al. International IBD Genetics Consortium (IIBDGC) . 2013. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 45:1353–1360. 10.1038/ng.2770 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

