Secreted Compounds of the Probiotic Bacillus clausii Strain O/C Inhibit the Cytotoxic Effects Induced by Clostridium difficile and Bacillus cereus Toxins
- PMID: 27001810
- PMCID: PMC4879374
- DOI: 10.1128/AAC.02815-15
Secreted Compounds of the Probiotic Bacillus clausii Strain O/C Inhibit the Cytotoxic Effects Induced by Clostridium difficile and Bacillus cereus Toxins
Abstract
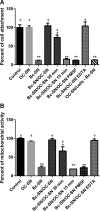
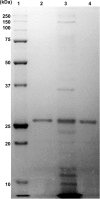
Although the use of probiotics based on Bacillus strains to fight off intestinal pathogens and antibiotic-associated diarrhea is widespread, the mechanisms involved in producing their beneficial effects remain unclear. Here, we studied the ability of compounds secreted by the probiotic Bacillus clausii strain O/C to counteract the cytotoxic effects induced by toxins of two pathogens, Clostridium difficile and Bacillus cereus, by evaluating eukaryotic cell viability and expression of selected genes. Coincubation of C. difficile and B. cereus toxic culture supernatants with the B. clausii supernatant completely prevented the damage induced by toxins in Vero and Caco-2 cells. The hemolytic effect of B. cereus was also avoided by the probiotic supernatant. Moreover, in these cells, the expression of rhoB, encoding a Rho GTPase target for C. difficile toxins, was normalized when C. difficile supernatant was pretreated using the B. clausii supernatant. All of the beneficial effects observed with the probiotic were abolished by the serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF). Suspecting the involvement of a secreted protease in this protective effect, a protease was purified from the B. clausii supernatant and identified as a serine protease (M-protease; GenBank accession number Q99405). Experiments on Vero cells demonstrated the antitoxic activity of the purified protease against pathogen supernatants. This is the first report showing the capacity of a protease secreted by probiotic bacteria to inhibit the cytotoxic effects of toxinogenic C. difficile and B. cereus strains. This extracellular compound could be responsible, at least in part, for the protective effects observed for this human probiotic in antibiotic-associated diarrhea.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Kefir-isolated Lactococcus lactis subsp. lactis inhibits the cytotoxic effect of Clostridium difficile in vitro.J Dairy Res. 2013 Feb;80(1):96-102. doi: 10.1017/S0022029912000623. Epub 2012 Dec 10. J Dairy Res. 2013. PMID: 23217732
-
Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa.Infect Immun. 1999 Jan;67(1):302-7. doi: 10.1128/IAI.67.1.302-307.1999. Infect Immun. 1999. PMID: 9864230 Free PMC article.
-
Difference in the cytotoxic effects of toxin B from Clostridium difficile strain VPI 10463 and toxin B from variant Clostridium difficile strain 1470.Infect Immun. 2007 Feb;75(2):801-9. doi: 10.1128/IAI.01705-06. Epub 2006 Dec 4. Infect Immun. 2007. PMID: 17145947 Free PMC article.
-
Theodore E. Woodward Award. How bacterial enterotoxins work: insights from in vivo studies.Trans Am Clin Climatol Assoc. 2002;113:167-80; discussion 180-1. Trans Am Clin Climatol Assoc. 2002. PMID: 12053708 Free PMC article. Review.
-
Mechanistic Insight into the Role of Peptides Secreted from Bacillus clausii and Future Opportunities.Curr Rev Clin Exp Pharmacol. 2024;19(4):379-386. doi: 10.2174/0127724328273252240201071756. Curr Rev Clin Exp Pharmacol. 2024. PMID: 38375835 Review.
Cited by
-
Fighting AMR in the Healthcare Environment: Microbiome-Based Sanitation Approaches and Monitoring Tools.Int J Mol Sci. 2019 Mar 27;20(7):1535. doi: 10.3390/ijms20071535. Int J Mol Sci. 2019. PMID: 30934725 Free PMC article. Review.
-
Probiotics L. acidophilus and B. clausii Modulate Gut Microbiota in Th1- and Th2-Biased Mice to Ameliorate Salmonella Typhimurium-Induced Diarrhea.Probiotics Antimicrob Proteins. 2019 Sep;11(3):887-904. doi: 10.1007/s12602-018-9436-5. Probiotics Antimicrob Proteins. 2019. PMID: 29909486
-
Current Progress and Future Perspectives on the Use of Bacillus clausii.Microorganisms. 2022 Jun 17;10(6):1246. doi: 10.3390/microorganisms10061246. Microorganisms. 2022. PMID: 35744764 Free PMC article. Review.
-
Protective Effects of Bifidobacterial Strains Against Toxigenic Clostridium difficile.Front Microbiol. 2018 May 8;9:888. doi: 10.3389/fmicb.2018.00888. eCollection 2018. Front Microbiol. 2018. PMID: 29867801 Free PMC article.
-
Strain-Dependent Adhesion Variations of Shouchella clausii Isolated from Healthy Human Volunteers: A Study on Cell Surface Properties and Potential Probiotic Benefits.Microorganisms. 2024 Aug 27;12(9):1771. doi: 10.3390/microorganisms12091771. Microorganisms. 2024. PMID: 39338446 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

