Representation of spontaneous movement by dopaminergic neurons is cell-type selective and disrupted in parkinsonism
- PMID: 27001837
- PMCID: PMC4839395
- DOI: 10.1073/pnas.1515941113
Representation of spontaneous movement by dopaminergic neurons is cell-type selective and disrupted in parkinsonism
Abstract
Midbrain dopaminergic neurons are essential for appropriate voluntary movement, as epitomized by the cardinal motor impairments arising in Parkinson's disease. Understanding the basis of such motor control requires understanding how the firing of different types of dopaminergic neuron relates to movement and how this activity is deciphered in target structures such as the striatum. By recording and labeling individual neurons in behaving mice, we show that the representation of brief spontaneous movements in the firing of identified midbrain dopaminergic neurons is cell-type selective. Most dopaminergic neurons in the substantia nigra pars compacta (SNc), but not in ventral tegmental area or substantia nigra pars lateralis, consistently represented the onset of spontaneous movements with a pause in their firing. Computational modeling revealed that the movement-related firing of these dopaminergic neurons can manifest as rapid and robust fluctuations in striatal dopamine concentration and receptor activity. The exact nature of the movement-related signaling in the striatum depended on the type of dopaminergic neuron providing inputs, the striatal region innervated, and the type of dopamine receptor expressed by striatal neurons. Importantly, in aged mice harboring a genetic burden relevant for human Parkinson's disease, the precise movement-related firing of SNc dopaminergic neurons and the resultant striatal dopamine signaling were lost. These data show that distinct dopaminergic cell types differentially encode spontaneous movement and elucidate how dysregulation of their firing in early Parkinsonism can impair their effector circuits.
Keywords: Parkinson's disease; alpha-synuclein; dopamine; substantia nigra; ventral tegmental area.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
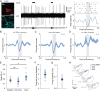
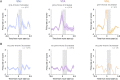

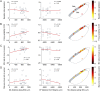

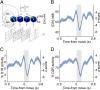




References
Publication types
MeSH terms
Substances
Grants and funding
- G0700932/MRC_/Medical Research Council/United Kingdom
- MC_UU_12024/2/MRC_/Medical Research Council/United Kingdom
- 101821/WT_/Wellcome Trust/United Kingdom
- G-1003/PUK_/Parkinson's UK/United Kingdom
- MC_U138164490/MRC_/Medical Research Council/United Kingdom
- MC_UU_12020/5/MRC_/Medical Research Council/United Kingdom
- G-0803/PUK_/Parkinson's UK/United Kingdom
- MR/K013866/1/MRC_/Medical Research Council/United Kingdom
- G-1103/PUK_/Parkinson's UK/United Kingdom
- MR/J004324/1/MRC_/Medical Research Council/United Kingdom
- J-0901/PUK_/Parkinson's UK/United Kingdom
- G-1305/PUK_/Parkinson's UK/United Kingdom
- G-0808/PUK_/Parkinson's UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

