Spatiotemporal dynamics of auditory attention synchronize with speech
- PMID: 27001861
- PMCID: PMC4833226
- DOI: 10.1073/pnas.1523357113
Spatiotemporal dynamics of auditory attention synchronize with speech
Abstract
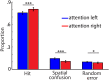
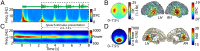
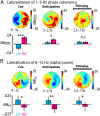
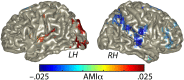
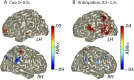
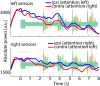
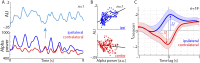
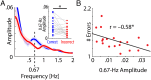
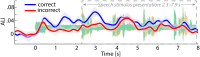
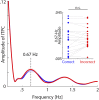
Attention plays a fundamental role in selectively processing stimuli in our environment despite distraction. Spatial attention induces increasing and decreasing power of neural alpha oscillations (8-12 Hz) in brain regions ipsilateral and contralateral to the locus of attention, respectively. This study tested whether the hemispheric lateralization of alpha power codes not just the spatial location but also the temporal structure of the stimulus. Participants attended to spoken digits presented to one ear and ignored tightly synchronized distracting digits presented to the other ear. In the magnetoencephalogram, spatial attention induced lateralization of alpha power in parietal, but notably also in auditory cortical regions. This alpha power lateralization was not maintained steadily but fluctuated in synchrony with the speech rate and lagged the time course of low-frequency (1-5 Hz) sensory synchronization. Higher amplitude of alpha power modulation at the speech rate was predictive of a listener's enhanced performance of stream-specific speech comprehension. Our findings demonstrate that alpha power lateralization is modulated in tune with the sensory input and acts as a spatiotemporal filter controlling the read-out of sensory content.
Keywords: alpha lateralization; attention; neural oscillations; speech; synchronization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cogn Sci. 2003;7(12):553–559. - PubMed
-
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. - PubMed
-
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport. 1998;9(17):3929–3933. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

