Three-Dimensional Visualization of the Tubular-Lamellar Transformation of the Internal Plastid Membrane Network during Runner Bean Chloroplast Biogenesis
- PMID: 27002023
- PMCID: PMC4863387
- DOI: 10.1105/tpc.15.01053
Three-Dimensional Visualization of the Tubular-Lamellar Transformation of the Internal Plastid Membrane Network during Runner Bean Chloroplast Biogenesis
Abstract
Chloroplast biogenesis is a complex process that is integrated with plant development, leading to fully differentiated and functionally mature plastids. In this work, we used electron tomography and confocal microscopy to reconstruct the process of structural membrane transformation during the etioplast-to-chloroplast transition in runner bean (Phaseolus coccineus). During chloroplast development, the regular tubular network of paracrystalline prolamellar bodies (PLBs) and the flattened porous membranes of prothylakoids develop into the chloroplast thylakoids. Three-dimensional reconstruction is required to provide us with a more complete understanding of this transformation. We provide spatial models of the bean chloroplast biogenesis that allow such reconstruction of the internal membranes of the developing chloroplast and visualize the transformation from the tubular arrangement to the linear system of parallel lamellae. We prove that the tubular structure of the PLB transforms directly to flat slats, without dispersion to vesicles. We demonstrate that the grana/stroma thylakoid connections have a helical character starting from the early stages of appressed membrane formation. Moreover, we point out the importance of particular chlorophyll-protein complex components in the membrane stacking during the biogenesis. The main stages of chloroplast internal membrane biogenesis are presented in a movie that shows the time development of the chloroplast biogenesis as a dynamic model of this process.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures



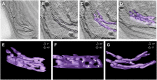
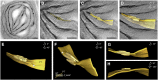


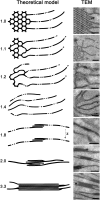

Comment in
-
3D Visualization of Thylakoid Membrane Development.Plant Cell. 2016 Apr;28(4):827-8. doi: 10.1105/tpc.16.00230. Epub 2016 Mar 21. Plant Cell. 2016. PMID: 27002024 Free PMC article. No abstract available.
References
-
- Abdelkader A.F., Aronsson H., Solymosi K., Böddi B., Sundqvist C. (2007). High salt stress induces swollen prothylakoids in dark-grown wheat and alters both prolamellar body transformation and reformation after irradiation. J. Exp. Bot. 58: 2553–2564. - PubMed
-
- Abramoff M.D., Magalhaes P.J., Ram S.J. (2004). Image processing with ImageJ. Biophotonics International. 11: 36–42.
-
- Adam Z., Charuvi D., Tsabari O., Knopf R.R., Reich Z. (2011). Biogenesis of thylakoid networks in angiosperms: knowns and unknowns. Plant Mol. Biol. 76: 221–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

