Allosteric Activation of Escherichia coli Glucosamine-6-Phosphate Deaminase (NagB) In Vivo Justified by Intracellular Amino Sugar Metabolite Concentrations
- PMID: 27002132
- PMCID: PMC4959280
- DOI: 10.1128/JB.00870-15
Allosteric Activation of Escherichia coli Glucosamine-6-Phosphate Deaminase (NagB) In Vivo Justified by Intracellular Amino Sugar Metabolite Concentrations
Abstract
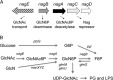
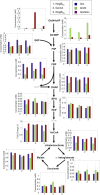
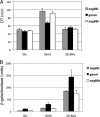
We have investigated the impact of growth on glucosamine (GlcN) and N-acetylglucosamine (GlcNAc) on cellular metabolism by quantifying glycolytic metabolites in Escherichia coli Growth on GlcNAc increased intracellular pools of both GlcNAc6P and GlcN6P 10- to 20-fold compared to growth on glucose. Growth on GlcN produced a 100-fold increase in GlcN6P but only a slight increase in GlcNAc6P. Changes to the amounts of downstream glycolytic intermediates were minor compared to growth on glucose. The enzyme glucosamine-6P deaminase (NagB) is required for growth on both GlcN and GlcNAc. It is an allosteric enzyme in E. coli, displaying sigmoid kinetics with respect to its substrate, GlcN6P, and is allosterically activated by GlcNAc6P. The high concentration of GlcN6P, accompanied by the small increase in GlcNAc6P, drives E. coli NagB (NagBEc) into its high activity state, as observed during growth on GlcN (L. I. Álvarez-Añorve, I. Bustos-Jaimes, M. L. Calcagno, and J. Plumbridge, J Bacteriol 191:6401-6407, 2009, http://dx.doi.org/10.1128/JB.00633-09). The slight increase in GlcNAc6P during growth on GlcN is insufficient to displace NagC, the GlcNAc6P-responsive repressor of the nag genes, from its binding sites, so there is only a small increase in nagB expression. We replaced the gene for the allosteric NagBEc enzyme with that of the nonallosteric, B. subtilis homologue, NagBBs We detected no effects on growth rates or competitive fitness on glucose or the amino sugars, nor did we detect any effect on the concentrations of central metabolites, thus demonstrating the robustness of amino sugar metabolism and leaving open the question of the role of allostery in the regulation of NagB.
Importance: Chitin, the polymer of N-acetylglucosamine, is an abundant biomaterial, and both glucosamine and N-acetylglucosamine are valuable nutrients for bacteria. The amino sugars are components of numerous essential macromolecules, including bacterial peptidoglycan and mammalian glycosaminoglycans. Controlling the biosynthetic and degradative pathways of amino sugar metabolism is important in all organisms to avoid loss of nitrogen and energy via a futile cycle of synthesis and breakdown. The enzyme glucosamine-6P deaminase (NagB) is central to this control, and N-acetylglucosamine-6P is the key signaling molecule regulating amino sugar utilization in Escherichia coli Here, we investigate how the metabolic status of the bacteria impacts on the activity of NagBEc and the N-acetylglucosamine-6P-sensitive transcriptional repressor, NagC.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

