Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria
- PMID: 27002652
- PMCID: PMC4804174
- DOI: 10.1038/ncomms11078
Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria
Abstract
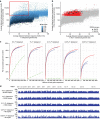
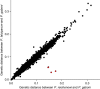
African apes harbour at least six Plasmodium species of the subgenus Laverania, one of which gave rise to human Plasmodium falciparum. Here we use a selective amplification strategy to sequence the genome of chimpanzee parasites classified as Plasmodium reichenowi and Plasmodium gaboni based on the subgenomic fragments. Genome-wide analyses show that these parasites indeed represent distinct species, with no evidence of cross-species mating. Both P. reichenowi and P. gaboni are 10-fold more diverse than P. falciparum, indicating a very recent origin of the human parasite. We also find a remarkable Laverania-specific expansion of a multigene family involved in erythrocyte remodelling, and show that a short region on chromosome 4, which encodes two essential invasion genes, was horizontally transferred into a recent P. falciparum ancestor. Our results validate the selective amplification strategy for characterizing cryptic pathogen species, and reveal evolutionary events that likely predisposed the precursor of P. falciparum to colonize humans.
Figures



Comment in
-
Unravelling the Laverania.Nat Rev Microbiol. 2016 Aug;14(8):478. doi: 10.1038/nrmicro.2016.109. Epub 2016 Jul 11. Nat Rev Microbiol. 2016. PMID: 27396565
References
-
- Bray R. S. The malaria parasites of anthropoid apes. J. Parasitol. 49, 888–891 (1963). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

