Long-term neuropathological and behavioral impairments after exposure to nerve agents
- PMID: 27002925
- PMCID: PMC4940270
- DOI: 10.1111/nyas.13028
Long-term neuropathological and behavioral impairments after exposure to nerve agents
Abstract
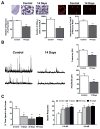
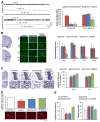
One of the deleterious effects of acute nerve agent exposure is the induction of status epilepticus (SE). If SE is not controlled effectively, it causes extensive brain damage. Here, we review the neuropathology observed after nerve agent-induced SE, as well as the ensuing pathophysiological, neurological, and behavioral alterations, with an emphasis on their time course and longevity. Limbic structures are particularly vulnerable to damage by nerve agent exposure. The basolateral amygdala (BLA), which appears to be a key site for seizure initiation upon exposure, suffers severe neuronal loss; however, GABAergic BLA interneurons display a delayed death, perhaps providing a window of opportunity for rescuing intervention. The end result is a long-term reduction of GABAergic activity in the BLA, with a concomitant increase in spontaneous excitatory activity; such pathophysiological alterations are not observed in the CA1 hippocampal area, despite the extensive neuronal loss. Hyperexcitability in the BLA may be at least in part responsible for the development of recurrent seizures and increased anxiety, while hippocampal damage may underlie the long-term memory impairments. Effective control of SE after nerve agent exposure, such that brain damage is also minimized, is paramount for preventing lasting neurological and behavioral deficits.
Keywords: anxiety; basolateral amygdala; hippocampus; nerve agents; seizures; status epilepticus.
© 2016 New York Academy of Sciences.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Bajgar J, Fusek J, Kassa J, et al. An attempt to assess functionally minimal acetylcholinesterase activity necessary for survival of rats intoxicated with nerve agents. Chem Biol Interact. 2008;175:281–285. - PubMed
-
- Shih TM, Kan RK, McDonough JH. In vivo cholinesterase inhibitory specificity of organophosphorus nerve agents. Chem Biol Interact. 2005;157–158:293–303. - PubMed
-
- Fountain NB. Status epilepticus: risk factors and complications. Epilepsia. 2000;41(Suppl 2):S23–30. - PubMed
-
- Schmued LC, Stowers CC, Scallet AC, et al. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

