Effect of Outer-Sphere Side Chain Substitutions on the Fate of the trans Iron-Nitrosyl Dimer in Heme/Nonheme Engineered Myoglobins (Fe(B)Mbs): Insights into the Mechanism of Denitrifying NO Reductases
- PMID: 27003474
- PMCID: PMC5181652
- DOI: 10.1021/acs.biochem.5b01109
Effect of Outer-Sphere Side Chain Substitutions on the Fate of the trans Iron-Nitrosyl Dimer in Heme/Nonheme Engineered Myoglobins (Fe(B)Mbs): Insights into the Mechanism of Denitrifying NO Reductases
Abstract
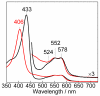
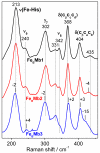
Denitrifying NO reductases are transmembrane protein complexes that utilize a heme/nonheme diiron center at their active sites to reduce two NO molecules to the innocuous gas N2O. Fe(B)Mb proteins, with their nonheme iron sites engineered into the heme distal pocket of sperm whale myoglobin, are attractive models for studying the molecular details of the NO reduction reaction. Spectroscopic and structural studies of Fe(B)Mb constructs have confirmed that they reproduce the metal coordination spheres observed at the active site of the cytochrome c-dependent NO reductase from Pseudomonas aeruginosa. Exposure of Fe(B)Mb to excess NO, as examined by analytical and spectroscopic techniques, results primarily in the formation of a five-coordinate heme-nitrosyl complex without N2O production. However, substitution of the outer-sphere residue Ile107 with a glutamic acid (i.e., I107E) decreases the formation rate of the five-coordinate heme-nitrosyl complex and allows for the substoichiometric production of N2O. Here, we aim to better characterize the formation of the five-coordinate heme-nitrosyl complex and to explain why the level of N2O production increases with the I107E substitution. We follow the formation of the five-coordinate heme-nitrosyl inhibitory complex through the sequential exposure of Fe(B)Mb to different NO isotopomers using rapid-freeze-quench resonance Raman spectroscopy. The data show that the complex is formed by the displacement of the proximal histidine by a new NO molecule after the weakening of the Fe(II)-His bond in the intermediate six-coordinate low-spin (6cLS) heme-nitrosyl complex. These results lead us to explore diatomic migration within the scaffold of myoglobin and whether substitutions at residue 107 can be sufficient to control access to the proximal heme cavities. Results on a new Fe(B)Mb construct with an I107F substitution (Fe(B)Mb3) show an increased rate for the formation of the five-coordinate low-spin heme-nitrosyl complex without N2O production. Taken together, our results suggest that production of N2O from the [6cLS heme {FeNO}(7)/{Fe(B)NO}(7)] trans iron-nitrosyl dimer intermediate requires a proton transfer event facilitated by an outer-sphere residue such as E107 in Fe(B)Mb2 and E280 in P. aeruginosa cNOR.
Figures





References
-
- Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vöosmarty CJ. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry. 2004;70:153–226.
-
- Stevanin TM, Laver JR, Poole RK, Moir JW, Read RC. Metabolism of nitric oxide by Neisseria meningitidis modifies release of NO-regulated cytokines and chemokines by human macrophages. Microbes. Infect. 2007;9:981–987. - PubMed
-
- Hino T, Matsumoto Y, Nagano S, Sugimoto H, Fukumori Y, Murata T, Iwata S, Shiro Y. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science. 2010;330:1666–1670. - PubMed
-
- Matsumoto Y, Tosha T, Pisliakov AV, Hino T, Sugimoto H, Nagano S, Sugita Y, Shiro Y. Crystal structure of quinol-dependent nitric oxide reductase from Geobacillus stearothermophilus. Nat. Struct. Mol. Biol. 2012;19:238–245. - PubMed
-
- Wasser IM, de Vries S, Moënne-Loccoz P, Schroder I, Karlin KD. Nitric oxide in biological denitrification: Fe/Cu metalloenzyme and metal complex NOx redox chemistry. Chem. Rev. 2002;102:1201–1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

