Treatment Patterns Associated with ACR-Recommended Medications in the Management of Fibromyalgia in the United States
- PMID: 27003556
- PMCID: PMC10398128
- DOI: 10.18553/jmcp.2016.22.3.263
Treatment Patterns Associated with ACR-Recommended Medications in the Management of Fibromyalgia in the United States
Abstract
Background: Fibromyalgia (FM) affects up to 6% of U.S. adults, resulting in a significant burden on the health care system and poor quality of life for patients. Duloxetine, pregabalin, and milnacipran are approved for management of FM; however, consensus is lacking regarding optimal therapy. Patients with FM taking approved medications often do not experience meaningful symptom relief, and many experience intolerable adverse events.
Objective: To assess treatment patterns associated with available and commonly used medications for the management of FM using U.S. health insurance claims.
Methods: This retrospective analysis used the MarketScan claims database to identify adults with a first diagnosis of FM (ICD-9-CM code 729.1) between 2009 and 2011 with continuous health plan enrollment for 12 months pre- and post-index. Medications of interest were pregabalin, gabapentin, duloxetine, milnacipran, cyclobenzaprine, and tramadol. These are 6 of the 8 medications recommended by the American College of Rheumatology (ACR) for treating FM; the other 2 (amitriptyline and venlafaxine) were only included in some initial assessments. The Charlson Comorbidity Index (CCI) was used to assess overall comorbidity burden. Endpoints included proportion of patients treated within 1 year after first diagnosis; initial treatment pattern; adherence over the first-year follow-up period for the medications of interest; and discontinuation, switching, and combination therapy patterns among pain medications of interest at different time points. Proportion of days covered (PDC; defined as number of days in the period when the patient had drug supply divided by the number of days in the period) was used to define adherence, which was categorized as low (PDC < 50%), medium (PDC 50% to < 80%), or high (PDC ≥ 80%). The time to discontinuation (defined as the first drug supply gap ≥ 90 days) was estimated using Kaplan-Meier analysis.
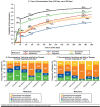
Results: Overall, 240,144 patients met the inclusion criteria. Patients were predominantly women (68%), had preferred provider organization insurance coverage (68%), and had a CCI score < 1 at baseline (69%). Only 31% (n = 74,738) initiated a treatment with a prescription medication listed in the ACR guidelines, and many patients received less than the recommended dose. Most (n = 70,919) patients initially received monotherapy with one of the 8 prescription medications. Of those who started with ≥ 2 medications (n = 3,819), cyclobenzaprine plus tramadol was the most frequent combination. Adherence was suboptimal for all 6 medications of interest. Duloxetine had the highest mean PDC (59%); for all other agents, mean PDC was < 50%. With the exception of duloxetine, discontinuation rates at 6 months were > 50% for all agents. Alterations in therapy were common. Among patients who discontinued their initial treatment of duloxetine, pregabalin, or milnacipran, approximately one-third had switched treatments within 90 days after their first prescription. For those who maintained their initial treatment agent, approximately 50% of patients added a second pain medication within 1 year of treatment initiation.
Conclusions: The evidence suggests that patients with FM often do not receive 1 of the prescription medications recommended by ACR guidelines, and those who do are commonly prescribed lower-than-recommended doses, potentially resulting in poor effectiveness and tolerability. Discontinuation, switching, and addition of new pain medications are common, which may indicate low levels of satisfaction with initial treatment. New therapies with improved effectiveness and better tolerability are urgently needed for patients with FM.
Conflict of interest statement
No outside funding supported this research. Yang was an employee of Daiichi Sankyo while this study was conducted. Qian is an employee of Daiichi Sankyo. Liu has nothing to disclose.
Yang, Qian, and Liu interpreted the data and critically reviewed and edited the paper. Qian provided the analyses. All authors had access to the data. Qian is the guarantor for this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
References
-
- Goldenberg DL, Clauw DJ, Fitzcharles MA. New concepts in pain research and pain management of the rheumatic diseases. Semin Arthritis Rheum. 2011;41(3):319-34. - PubMed
-
- Centers for Disease Control and Prevention . Fibromyalgia. Available at: http://www.cdc.gov/arthritis/basics/fibromyalgia.htm. Accessed December 13, 2015.
-
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17(8):356. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous