Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations
- PMID: 27004086
- PMCID: PMC4794528
- DOI: 10.4254/wjh.v8.i8.385
Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations
Abstract
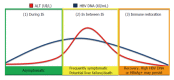
The proportion of hepatitis B virus (HBV) previously exposed patients who receive immunosuppressive treatment is usually very small. However, if these individuals are exposed to potent immunosuppressive compounds, the risk of HBV reactivation (HBVr) increases with the presence of hepatitis B surface antigen (HBsAg) in the serum. Chronic HBsAg carriers have a higher risk than those who have a total IgG anticore as the only marker of resolved/occult HBV disease. The loss of immune control in these patients may results in the reactivation of HBV replication within hepatocytes. Upon reconstitution of the immune system, infected hepatocytes are once again targeted and damaged by immune surveillance in an effort to clear the virus. There are different virological scenarios, and a wide spectrum of associated drugs with specific and stratified risk for the development of HBVr. Some of this agents can trigger a severe degree of hepatocellular damage, including hepatitis, acute liver failure, and even death despite employment of effective antiviral therapies. Currently, HBVr incidence seems to be increasing around the world; a fact mainly related to the incessant appearance of more powerful immunosuppressive drugs launched to the market. Moreover, there is no consensus on the length of prophylactic treatment before the patients are treated with immunosuppressive therapy, and for how long this therapy should be extended once treatment is completed. Therefore, this review article will focus on when to treat, when to monitor, what patients should receive HBV therapy, and what drugs should be selected for each scenario. Lastly, we will update the definition, risk factors, screening, and treatment recommendations based on both current and different HBV management guidelines.
Keywords: Acute liver failure; Anti-tumor necrosis factor-α drugs; Biologic therapy; Hepatitis B; Immunosuppressive therapy.
Figures


References
-
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. - PubMed
-
- Roche B, Samuel D. The difficulties of managing severe hepatitis B virus reactivation. Liver Int. 2011;31 Suppl 1:104–110. - PubMed
-
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

