Biodegradable stents for the treatment of bowel strictures in Crohn's disease: technical results and challenges
- PMID: 27004247
- PMCID: PMC4798842
- DOI: 10.1055/s-0042-101940
Biodegradable stents for the treatment of bowel strictures in Crohn's disease: technical results and challenges
Abstract
Background and study aims: In patients with Crohn's disease, the idea of biodegradable stents for treatment of bowel strictures with limited effect of endoscopic balloon dilation is tempting and initial results have been promising. The aim of this study was to evaluate the technical and clinical success of biodegradable stents for treatment of inflamed Crohn's strictures refractory to endoscopic balloon dilatation.
Patients and methods: Consecutive patients treated with biodegradable stents due to Crohn's disease and inflamed bowel strictures refractory to endoscopic balloon dilatation were included. Technical and clinical success were evaluated.
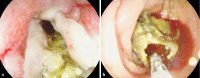
Results: Six patients were included in the study. Technical success was obtained in five patients (83 %). Clinical success was limited to one patient (20 %); failure was observed due to mucosal overgrowth (n = 2), stent migration (n = 1), and stent collapse (n = 1).
Conclusions: In Crohn's disease, it is technically feasible to treat bowel strictures with biodegradable stents. However, we have stopped using biodegradable stents due to lack of clinical success and side effects such as mucosal overgrowth and stent collapse.
Conflict of interest statement
Figures


References
-
- Dignass A, Van Assche G, Lindsay J O. et al.The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Current management. J Crohn's Colitis. 2010;4:28–62. - PubMed
-
- Thienpont C, D'Hoore A, Vermeire S. et al.Long-term outcome of endoscopic dilatation in patients with Crohn's disease is not affected by disease activity or medical therapy. Gut. 2010;59:320–324. - PubMed
-
- Karstensen J G, Hendel J, Vilmann P. Endoscopic balloon dilatation for Crohn's strictures of the gastrointestinal tract is feasible. Dan Med J. 2012;59:A4471. - PubMed
-
- Ferlitsch A, Reinisch W, Puspok A. et al.Safety and efficacy of endoscopic balloon dilation for treatment of Crohn's disease strictures. Endoscopy. 2006;38:483–487. - PubMed
-
- Attar A, Maunoury V, Vahedi K. et al.Safety and efficacy of extractible self-expandable metal stents in the treatment of Crohn's disease intestinal strictures: a prospective pilot study. Inflamm Bowel Dis. 2012;18:1849–1854. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

