Perioperative hemodynamic optimization using the photoplethysmography in colorectal surgery (the PANEX3 trial): study protocol for a randomized controlled trial
- PMID: 27004412
- PMCID: PMC4804484
- DOI: 10.1186/s13063-016-1278-4
Perioperative hemodynamic optimization using the photoplethysmography in colorectal surgery (the PANEX3 trial): study protocol for a randomized controlled trial
Abstract
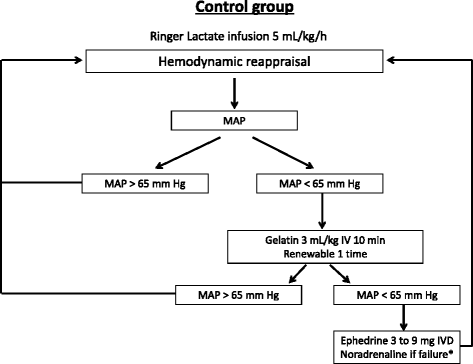
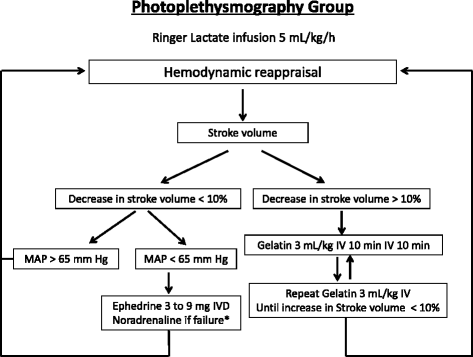
Background: Photoplethysmography with a digital sensor (ClearSight, Edwards Lifesciences, Irvine, CA, USA) connected to a dedicated monitor (EV 1000, Edwards Lifesciences) was recently proposed for use in performing hemodynamic optimization during surgery. The objective of this study is to evaluate the effect of photoplethysmography on the incidence of postoperative complications compared with the conventional hemodynamic algorithm, which uses mean arterial pressure.
Methods/design: The hemodynamic optimization using photoplethysmography (PANEX3) trial is a monocentric, randomized, single-blind, controlled, two parallel arm, superiority trial, randomizing 160 patients with an intermediate risk of postoperative complications after colorectal surgery. Informed consent will be obtained from all participants. The hemodynamic optimization is conducted using a specified hemodynamic algorithm either with photoplethysmography (the photoplethysmography group) or with conventional mean arterial pressure (the control group). The anesthesiologist performed a 1:1 randomization the day before surgery using a scratch card, which is available 24/7. The randomization sequence is generated using permutated blocks. Both the patients and surgeons are blinded to the allocation group. The primary outcome is the incidence of at least one postoperative complication during the 30 days following surgery. Two independent experts, who were blinded to the group allocations, validate the complication for each patient using an a priori classification. The secondary outcomes are to study the total number of postoperative complications, the real length of hospital stays, and the postoperative mortality between each group.
Discussion: The PANEX3 trial is the first randomized controlled study conducted to investigate whether perioperative hemodynamic optimization using photoplethysmography during colorectal surgery could decrease the incidence of patients having at least one postoperative complication.
Trial registration: ClinicalTrials.gov Identifier: NCT02343601.
Keywords: Abdominal surgery; Anesthesiology; Hemodynamic; Plethysmography.
Figures

References
-
- Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. European Surgical Outcomes Study (EuSOS) group for the Trials groups of the European Society of Intensive Care Medicine and the European Society of Anaesthesiology. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–65. doi: 10.1016/S0140-6736(12)61148-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

