Tuberculosis incidence is high in HIV-infected African children but is reduced by co-trimoxazole and time on antiretroviral therapy
- PMID: 27004529
- PMCID: PMC4804479
- DOI: 10.1186/s12916-016-0593-7
Tuberculosis incidence is high in HIV-infected African children but is reduced by co-trimoxazole and time on antiretroviral therapy
Abstract
Background: There are few data on tuberculosis (TB) incidence in HIV-infected children on antiretroviral therapy (ART). Observational studies suggest co-trimoxazole prophylaxis may prevent TB, but there are no randomized data supporting this. The ARROW trial, which enrolled HIV-infected children initiating ART in Uganda and Zimbabwe and included randomized cessation of co-trimoxazole prophylaxis, provided an opportunity to estimate the incidence of TB over time, to explore potential risk factors for TB, and to evaluate the effect of stopping co-trimoxazole prophylaxis.
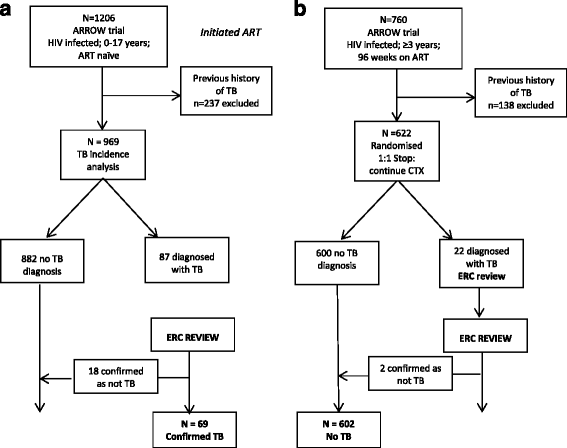
Methods: Of 1,206 children enrolled in ARROW, there were 969 children with no previous TB history. After 96 weeks on ART, children older than 3 years were randomized to stop or continue co-trimoxazole prophylaxis; 622 were eligible and included in the co-trimoxazole analysis. Endpoints, including TB, were adjudicated blind to randomization by an independent endpoint review committee (ERC). Crude incidence rates of TB were estimated and potential risk factors, including age, sex, center, CD4, weight, height, and initial ART strategy, were explored in multivariable Cox proportional hazards models.
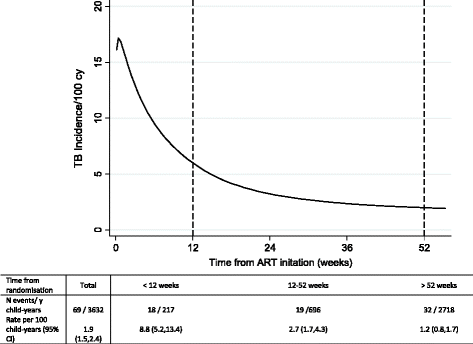
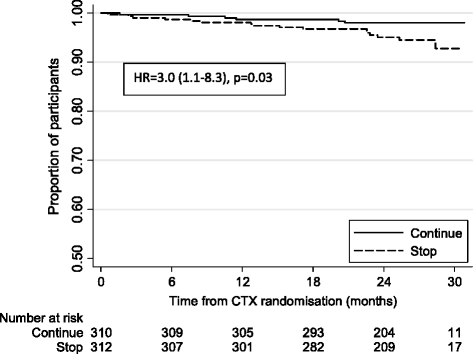
Results: After a median of 4 years follow-up (3,632 child-years), 69 children had an ERC-confirmed TB diagnosis. The overall TB incidence was 1.9/100 child-years (95% CI, 1.5-2.4), and was highest in the first 12 weeks following ART initiation (8.8/100 child-years (5.2-13.4) versus 1.2/100 child-years (0.8-1.6) after 52 weeks). A higher TB risk was independently associated with younger age (<3 years), female sex, lower pre-ART weight-for-age Z-score, and current CD4 percent; fewer TB diagnoses were observed in children on maintenance triple nucleoside reverse transcriptase inhibitor (NRTI) ART compared to standard non-NRTI + 2NRTI. Over the median 2 years of follow-up, there were 20 ERC-adjudicated TB cases among 622 children in the co-trimoxazole analysis: 5 in the continue arm and 15 in the stop arm (hazard ratio (stop: continue) = 3.0 (95% CI, 1.1-8.3), P = 0.028). TB risk was also independently associated with lower current CD4 percent (P <0.001).
Conclusions: TB incidence varies over time following ART initiation, and is particularly high during the first 3 months post-ART, reinforcing the importance of TB screening prior to starting ART and use of isoniazid preventive therapy once active TB is excluded. HIV-infected children continuing co-trimoxazole prophylaxis after 96 weeks of ART were diagnosed with TB less frequently, highlighting a potentially important role of co-trimoxazole in preventing TB.
Keywords: Antiretroviral therapy; HIV; Incident tuberculosis; Pediatric.
Figures
References
-
- World Health Organization . Global Tuberculosis Report. Geneva: WHO; 2015.
-
- World Health Organization . Global Tuberculosis Report. Geneva: WHO; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials