Immunogenicity of a Trivalent Recombinant Vaccine Against Clostridium perfringens Alpha, Beta, and Epsilon Toxins in Farm Ruminants
- PMID: 27004612
- PMCID: PMC4804304
- DOI: 10.1038/srep22816
Immunogenicity of a Trivalent Recombinant Vaccine Against Clostridium perfringens Alpha, Beta, and Epsilon Toxins in Farm Ruminants
Abstract
Clostridium perfringens is an anaerobic bacterium that produces several toxins. Of these, the alpha, beta, and epsilon toxins are responsible for causing the most severe C. perfringens-related diseases in farm animals. The best way to control these diseases is through vaccination. However, commercially available vaccines are based on inactivated toxins and have many production drawbacks, which can be overcome through the use of recombinant antigens. In this study, we produced recombinant alpha, beta, and epsilon toxins in Escherichia coli to formulate a trivalent vaccine. Its effectiveness was evaluated through a potency test in rabbits, in which the vaccine generated 9.6, 24.4, and 25.0 IU/mL of neutralizing antibodies against the respective toxins. Following this, cattle, sheep, and goats received the same formulation, generating, respectively, 5.19 ± 0.48, 4.34 ± 0.43, and 4.70 ± 0.58 IU/mL against alpha toxin, 13.71 ± 1.17 IU/mL (for all three species) against beta toxin, and 12.74 ± 1.70, 7.66 ± 1.69, and 8.91 ± 2.14 IU/mL against epsilon toxin. These levels were above the minimum recommended by international protocols. As such, our vaccine was effective in generating protective antibodies and, thus, may represent an interesting alternative for the prevention of C. perfringens-related intoxications in farm animals.
Figures
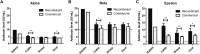
Similar articles
-
Potential protective immunogenicity of recombinant Clostridium perfringens α-β2-β1 fusion toxin in mice, sows and cows.Vaccine. 2011 Jul 26;29(33):5459-66. doi: 10.1016/j.vaccine.2011.05.059. Epub 2011 Jun 12. Vaccine. 2011. PMID: 21641956
-
Potency against enterotoxemia of a recombinant Clostridium perfringens type D epsilon toxoid in ruminants.Vaccine. 2010 Aug 31;28(38):6125-7. doi: 10.1016/j.vaccine.2010.07.046. Epub 2010 Jul 29. Vaccine. 2010. PMID: 20670910
-
Measurement over 1 Year of Neutralizing Antibodies in Cattle Immunized with Trivalent Vaccines Recombinant Alpha, Beta and Epsilon of Clostridium perfringens.Toxins (Basel). 2021 Aug 26;13(9):594. doi: 10.3390/toxins13090594. Toxins (Basel). 2021. PMID: 34564599 Free PMC article.
-
Vaccines against Clostridium perfringens alpha-toxin.Curr Pharm Biotechnol. 2013;14(10):913-7. doi: 10.2174/1389201014666131226124348. Curr Pharm Biotechnol. 2013. PMID: 24372250 Review.
-
Recombinant Alpha, Beta, and Epsilon Toxins of Clostridium perfringens: Production Strategies and Applications as Veterinary Vaccines.Toxins (Basel). 2016 Nov 21;8(11):340. doi: 10.3390/toxins8110340. Toxins (Basel). 2016. PMID: 27879630 Free PMC article. Review.
Cited by
-
Complete genomic sequence and analysis of β2 toxin gene mapping of Clostridium perfringens JXJA17 isolated from piglets in China.Sci Rep. 2021 Jan 12;11(1):475. doi: 10.1038/s41598-020-79333-8. Sci Rep. 2021. PMID: 33436645 Free PMC article.
-
Design of a chitosan-based nano vaccine against epsilon toxin of Clostridium perfringens type D and evaluation of its immunogenicity in BALB/c mice.Res Pharm Sci. 2021 Oct 15;16(6):575-585. doi: 10.4103/1735-5362.327504. eCollection 2021 Dec. Res Pharm Sci. 2021. PMID: 34760006 Free PMC article.
-
Clostridial Infections in Cattle: A Comprehensive Review with Emphasis on Current Data Gaps in Brazil.Animals (Basel). 2024 Oct 10;14(20):2919. doi: 10.3390/ani14202919. Animals (Basel). 2024. PMID: 39457848 Free PMC article. Review.
-
Humoral Immune Response Evaluation in Horses Vaccinated with Recombinant Clostridium perfringens Toxoids Alpha and Beta for 12 Months.Toxins (Basel). 2021 Aug 13;13(8):566. doi: 10.3390/toxins13080566. Toxins (Basel). 2021. PMID: 34437437 Free PMC article.
-
Vaccination against pathogenic clostridia in animals: a review.Trop Anim Health Prod. 2021 Apr 23;53(2):284. doi: 10.1007/s11250-021-02728-w. Trop Anim Health Prod. 2021. PMID: 33891221 Free PMC article. Review.
References
-
- Uzal F. A. & Songer J. G. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. J. Vet. Diagn. Invest. 20, 253–65 (2008). - PubMed
-
- Titball R. W. Clostridium perfringens vaccines. Vaccine 27, 44–47 (2009). - PubMed
-
- Stevens D. L. & Bryant A. E. The Role of Clostridial Toxins in the Pathogenesis of Gas Gangrene. Clin. Infect. Dis. 83702, 93–100 (2002). - PubMed
-
- Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Available at: http://www.agricultura.gov.br/animal. (Acessed: 27th November 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

