Flow cytometry analysis of the microbiota associated with the midguts of vector mosquitoes
- PMID: 27004717
- PMCID: PMC4802834
- DOI: 10.1186/s13071-016-1438-0
Flow cytometry analysis of the microbiota associated with the midguts of vector mosquitoes
Abstract
Background: The scientific interest to understand the function and structure of the microbiota associated with the midgut of mosquito disease vectors is increasing. The advancement of such a knowledge has encountered challenges and limitations associated with conventional culture-based and PCR techniques.
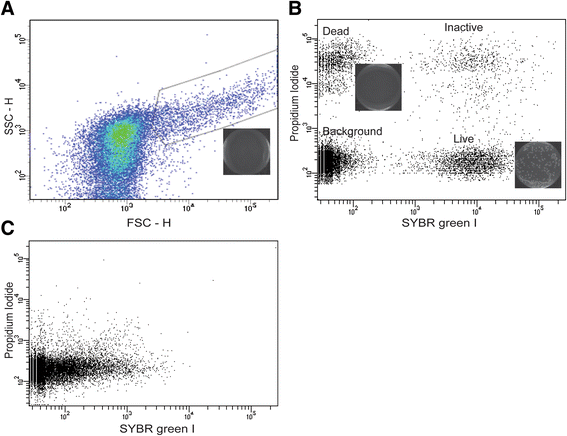
Methods: Flow cytometry (FCM) combined with various cell marking dyes have been successfully applied in the field of ecological microbiology to circumvent the above shortcomings. Here, we describe FCM technique coupled with live/dead differential staining dyes SYBR Green I (SGI) and Propidium Iodide (PI) to quantify and study other essential characteristics of the mosquito gut microbiota.
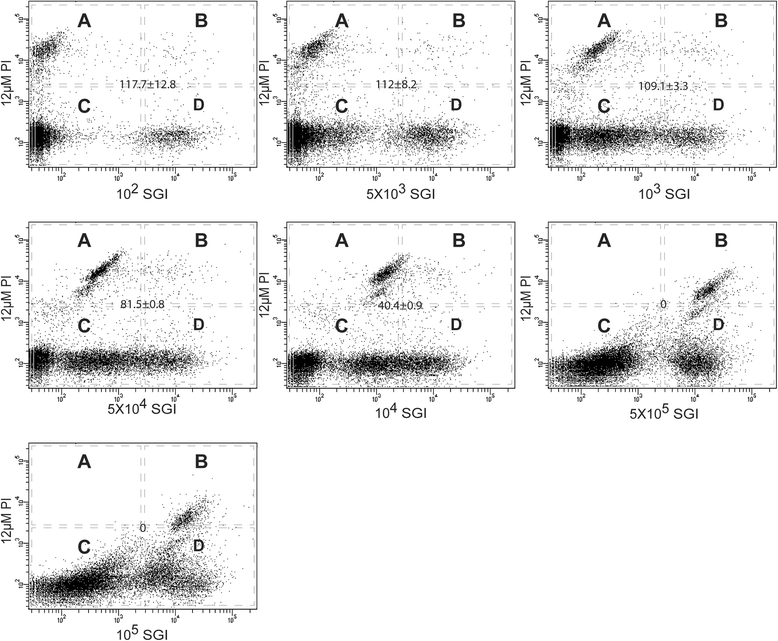
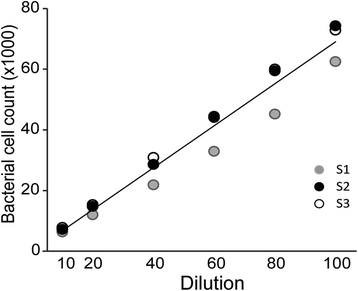
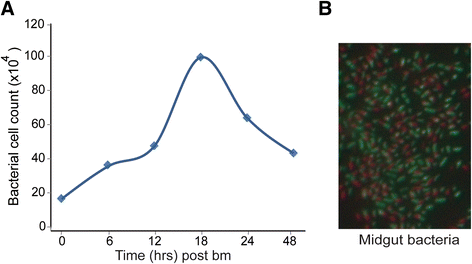
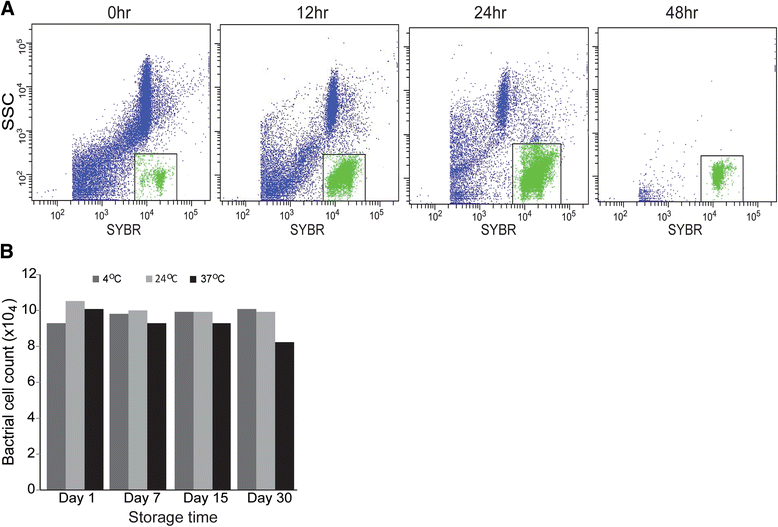
Results: A clear discrimination between cells and debris, as well as between live and dead cells was achieved when the midgut homogenate was subjected to staining with 5 × 103 dilution of the SGI and 30 μM concentration of the PI. Reproducibly, FCM event collections produced discrete populations including non-fluorescent cells, SYBR positive cells, PI fluorescing cells and cells that fluoresce both in SYBR and PI, all these cell populations representing, respectively, background noise, live bacterial, dead cells and inactive cells with partial permeability to PI. The FCM produced a strong linear relationship between cell counts and their corresponding dilution factors (R (2) = 0.987), and the technique has a better precision compared to qRT-PCR. The FCM count of the microbiota reached a peak load at 18 h post-feeding and started declining at 24 h. The present FCM technique also successfully applied to quantify bacterial cells in fixed midgut samples that were homogenized in 4 % PFA.
Conclusion: The FCM technique described here offers enormous potential and possibilities of integration with advanced molecular biochemical techniques for the study of the microbiota community in disease vector mosquitoes.
Keywords: Anopheles Coluzzii; Dead; Fixed cells; Flow cytometry; Live; Microbiota; Midgut homogenate; Propidium Iodide; discrimination.
Figures


References
-
- Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC. Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg. 1996;54(2):214–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

