Constitutive aneuploidy and genomic instability in the single-celled eukaryote Giardia intestinalis
- PMID: 27004936
- PMCID: PMC4985590
- DOI: 10.1002/mbo3.351
Constitutive aneuploidy and genomic instability in the single-celled eukaryote Giardia intestinalis
Abstract
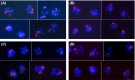
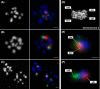
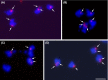
Giardia intestinalis is an important single-celled human pathogen. Interestingly, this organism has two equal-sized transcriptionally active nuclei, each considered diploid. By evaluating condensed chromosome numbers and visualizing homologous chromosomes by fluorescent in situ hybridization, we determined that the Giardia cells are constitutively aneuploid. We observed karyotype inter-and intra-population heterogeneity in eight cell lines from two clinical isolates, suggesting constant karyotype evolution during in vitro cultivation. High levels of chromosomal instability and frequent mitotic missegregations observed in four cell lines correlated with a proliferative disadvantage and growth retardation. Other cell lines, although derived from the same clinical isolate, revealed a stable yet aneuploid karyotype. We suggest that both chromatid missegregations and structural rearrangements contribute to shaping the Giardia genome, leading to whole-chromosome aneuploidy, unequal gene distribution, and a genomic divergence of the two nuclei within one cell. Aneuploidy in Giardia is further propagated without p53-mediated cell cycle arrest and might have been a key mechanism in generating the genetic diversity of this human pathogen.
Keywords: Aneuploidy; FISH; chromosome; giardia; karyotype; protist.
© 2016 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures




References
-
- Adam, R. D. , Dahlstrom E. W., Martens C. A., Bruno D. P., Barbian K. D., Ricklefs S. M., et al. 2013. Genome sequencing of Giardia lamblia genotypes A2 and B isolates (DH and GS) and comparative analysis with the genomes of genotypes A1 and E (WB and Pig). Genome Biol. Evol. 5:2498–2511. - PMC - PubMed
-
- Agresti, A . 2002. Appendix B: Chi‐Squared Distribution Values Pp. 654–654 in Categorical Data Analysis. John Wiley & Sons, Inc., Hoboken, New Jersey.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

